Honors Chemistry Written Final Exam Practice Test
advertisement
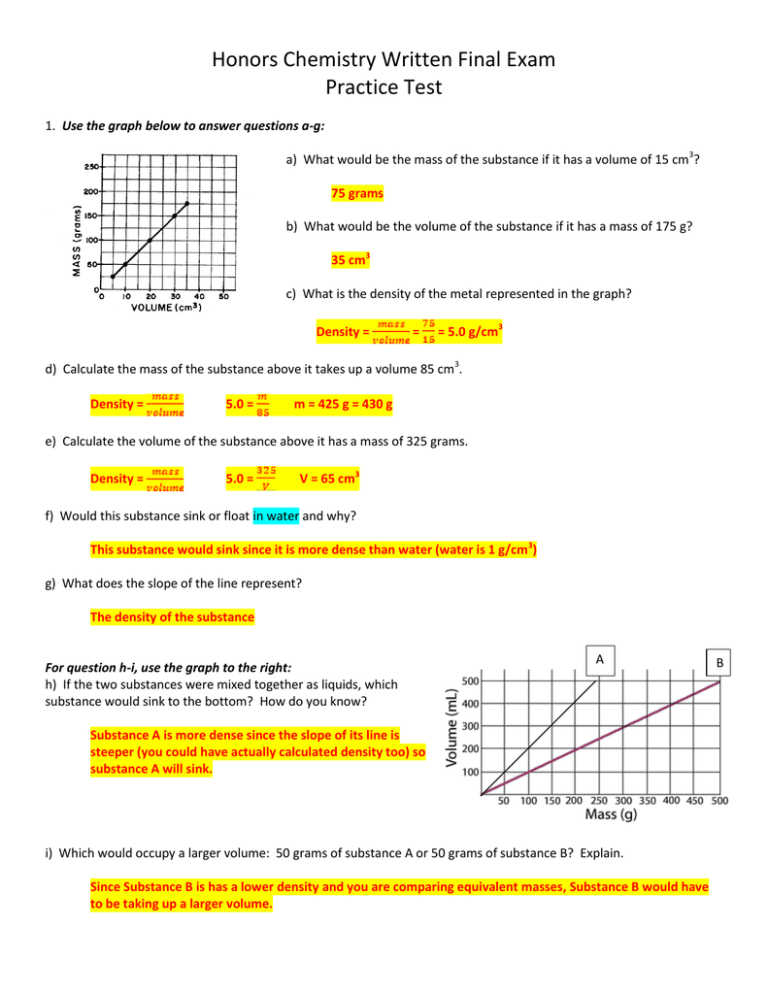
Honors Chemistry Written Final Exam Practice Test 1. Use the graph below to answer questions a-g: a) What would be the mass of the substance if it has a volume of 15 cm3? 75 grams b) What would be the volume of the substance if it has a mass of 175 g? 35 cm3 c) What is the density of the metal represented in the graph? Density = = = 5.0 g/cm3 d) Calculate the mass of the substance above it takes up a volume 85 cm3. Density = 5.0 = m = 425 g = 430 g e) Calculate the volume of the substance above it has a mass of 325 grams. Density = 5.0 = V = 65 cm3 f) Would this substance sink or float in water and why? This substance would sink since it is more dense than water (water is 1 g/cm3) g) What does the slope of the line represent? The density of the substance For question h-i, use the graph to the right: h) If the two substances were mixed together as liquids, which substance would sink to the bottom? How do you know? A Substance A is more dense since the slope of its line is steeper (you could have actually calculated density too) so substance A will sink. i) Which would occupy a larger volume: 50 grams of substance A or 50 grams of substance B? Explain. Since Substance B is has a lower density and you are comparing equivalent masses, Substance B would have to be taking up a larger volume. B 2. strontium + tin(II) sulfate a) Write the formulas for the reactants __Sr + SnSO4_____________ b) Write the names of the products ___strontium sulfate and tin__________________ c) Write the formulas for the products _ SrSO4 + Sn______________________________________ d) Write the balanced equation _ Sr + SnSO4--> SrSO4 + Sn _______________________ e) What is the oxidation state (charge) for each of the cations? _Sn+2 and Sr+2_______________ f) What is the oxidation state (charge) of the anion? _SO4-2__________________ g) How many sulfurs are on the products side? __1___________ h) What is the electron dot structure of sulfur? _:S:________ i) What type of reactions is this? _single replacement_____________________________ 3. ammonium carbonate + sodium nitrate a) Write the formulas for the reactants __(NH4)2CO3 + NaNO3____________________________________ b) Write the names of the products _sodium carbonate and ammonium nitrate ____________________ c) Write the formulas for the products _Na2CO3 + NH4NO3________________________________________ d) Write the balanced equation _(NH4)2CO3 + 2NaNO3 Na2CO3 + 2NH4NO3______ e) What is the oxidation state (charge) for each of the cations? _NH4+1 and Na+1_________________ f) What is the oxidation state (charge) for each of the anions? _CO3-2 and NO3-1________________________ g) How many sodiums are on the products side? _2_______ h) What is the electron dot structure of sodium? _Na________ i) What type of reactions is this? __double replacement________