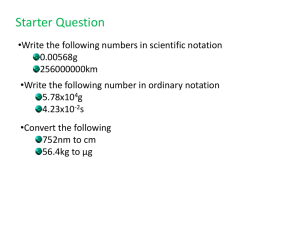
Chapter 1 (Part 2) Measurements in Chemistry 1.7 Physical
advertisement

Chapter 1 (Part 2) Measurements in Chemistry 1.7 Physical Quantities English Units Those of us who were raised in the US are very accustomed to these. Elsewhere in the world, these are very confusing. Weight: ounce (oz) pound (lb) [16 ounces = 1 pound] ton [2000 pounds = 1 ton] Notice that the English system of units uses ounces to describe both weight and volume measurements, which adds to the confusion. Length: inch (in) foot (ft) [12 inches = 1 foot] yard (yd) [3 feet = 1 yard] mile (mi) [5280 feet = 1 mile] Volume: teaspoon (tsp) tablespoon (Tbsp) [3 tsp = 1 Tbsp] cup [16 Tbsp = 1 cup = 8 oz] pint (pt) [2 cups = 1 pint = 16 oz] quart (qt) [4cups = 2 pints = 1 quart = 32 oz] gallon (gal) [4 quarts = 1 gallon=64oz] SI Units The scientific community has chosen a modified version of the metric system as the standard for recording and reporting measurements. Designated as SI (Systeme International) or International System of Units Some SI Base Units Measurement Name of Unit Abbr. Mass kilogram kg Length meter m Time second s Temperature Kelvin K Amount of substance mole mol Derived Units Volume Energy cubic decimeter = liter Joule Chapter 1 (Part 2) Page 1 of 22 dm3=10-3m3 L = dm3 J=kg x m2/sec2 Unit Prefixes Selected Prefixes in the Metric System Know by heart Prefix Abbr. Means Examples mega M 106 kilodecicentimillimicronano- k d c m n 103 10-1 10-2 10-3 10-6 10-9 pico- p 10-12 1 Megawatt = 1,000,000 watts 1 kilogram = 1,000 grams 1 dL = 0.1 liters 1 cm = 0.01 meters 1 mg = 0.001 grams 1 microliter = 0.000001 liters 1nanometer = 0.00000001 meters 1 picometer = 0.000000000001 meters 1,000,000 watt = 1 Mwatt 1000g = 1 kg 10 dL = 1 L 102 or 100 cm = 1 m 103 or 1000 mg = 1 g 106 L = 1L 109 nm = 1 m 1012 pm= 1 m Metric System This is a slight variation on the SI units of measure that is in common use in most countries other than the United States. Unit of mass is the gram (g) rather than the kilogram (1kg =1000g). Unit of volume is the liter (L) instead of the cubic meter (1 m3 = 1000L). Unit of temperature is the Celsius degree (C) rather than the Kelvin. Also known as the centigrade degree. Chapter 1 (Part 2) Page 2 of 22 1.8 Measuring Mass, Length, & Volume Mass Measurements The terms mass and weight are often confused and interchanged. • Mass - A measure of the amount of matter in an object. (how much stuff is present) • Weight - A measure of the gravitational force that the earth or other large body exerts on an object. e.g. We “weigh” less on the moon than on earth, but we have the same mass. Most useful: 1 lb = 16 oz = 454g 1 kg = 2.205 lb Length, Area and Volume Measurements For Length: Most useful: 1 inch = 2.54 cm exactly For Areas, that is easy to see: A square meter (m2) is an area 1 meter on each side. For volumes this is also true, but the final unit is often given a new name. A cubic centimeter (cm3 or cc) is an area 1 cm on each side = 1mL = 1cc A cubic decimeter (dm3) is an area 1 dm on each side = 1 L Chapter 1 (Part 2) Page 3 of 22 Most useful: 1 L = 1.057 qt 32 oz = 1 qt 1.9 Measurement and Significant Figures Numbers vs Data – Significant Figures In math class , numbers are theoretical, exact species. In science, most numbers are associated with measurements. Uncertainty of Data All measurements contain some uncertainty. We make errors Tools have limits We need to be able to show what degree of confidence we have in a piece of data. • The value recorded should use all the digits known with certainty, plus one estimated digit. Chapter 1 (Part 2) Page 4 of 22 • Significant figures – The number of meaningful digits used to express a value. Determining Significant Digits Last digit is uncertain Non-zero digits are ALWAYS significant. Zeros depend on whether they are leading, captive, or trailing LEADING zeroes are NEVER significant IMBEDDED zeros are ALWAYS significant TRAILING zeroes Depend after a decimal point ARE significant at the end of a # with no decimal point – we can’t tell Numbers with decimal points will be considered to be significant. (Confusion can be avoided by using scientific notation) Numbers that are definitions (e.g. 1 gallon = 4 quarts) have an infinite number of significant figures. Anything that gets counted in integer values is treated as exact. Problem: How many significant figures are in: a) 4009 b) 0.0455 c) 2806.0 d) 0.8904 e) 27.401 f) 4200. g) 4200 Chapter 1 (Part 2) Page 5 of 22 1.10 Scientific Notation Scientific notation is typically used to express very large or very small numbers or to clarify the number of significant digits present Expressed as N x 10n N is the digit term and is a number between 1 and 10. n is the exponential term. In scientific notation, all digits are significant. When converting a number to scientific notation: For every place you move the decimal to the left, add a power of 10. Example: 1 2 3 , 0 0 0 , 0 0 0 = 1.23 x 108 (positive powers of 10 are for BIG numbers) For every place you move the decimal to the Right, subtract a power of 10. Example: 0 . 0 0 0 0 0 0 1 2 3 = 1.23 x 10-7 (negative powers of 10 are for SMALL numbers) Chapter 1 (Part 2) Page 6 of 22 Problem: Write the following in scientific notation: a) 38666 b) 0.00407 c) 1300 to 2 sf d) 1300 to 3 sf e) 0.0000590 We also need to be able to convert values that are shown in scientific notation back to standard notation. Problem: Write the following in standard notation: a) 4.85 x 10-3 b) 3.270 x 103 c) 3.270 x 102 d) 8.819 x 10-6 e) 4.5500 x 103 1.11 Rounding Off Numbers Calculators often display more digits than are justified by the precision of the data. The last digit to be retained is increased by one if the following digit is 5 or greater. (i.e., 5,6,7,8, or 9) Example: 0.57266 rounded to 2 sf = The last digit to be retained is left unchanged if the following digit is 4 or less. (i.e., 0,1,2,3, or 4) Example: 0.57266 rounded to 3 sf = Chapter 1 (Part 2) Page 7 of 22 Round off the following numbers to the correct number of significant figures: Raw # Sig Figs Rounded In sci. not. 1.6753 1.6753 1.6753 1099.7 1099.7 1099.7 In the second example, we cannot tell once the number is rounded, how many significant figures the number represents. Scientific Notation (and Sig Figs) on Calculators When calculators display numbers in scientific notation, the display may show an E followed by a number 10x simply a number set off to the right side. Chapter 1 (Part 2) Page 8 of 22 To change a value to scientific notation: Using your calculator, convert the following to scientific notation: a) 38666 3.8666 x 104 b) 0.00407 4.07 x 10-4 c) 1300 to 2 sf 1.3 x 103 d) 1300 to 3 sf 1.30 x 103 To change a value to standard notation: Using your calculator, convert the following to standard notation: a) 4.85 x 10-3 0.00485 b) 3.270 x 103 3270. (decimal is import) c) 3.270 x 102 327.0 (zero is import) d) 8.819 x 10-6 0.000008819 e) 4.5500 x 103 4550.0 Chapter 1 (Part 2) Page 9 of 22 Calculators do not give us significant figures. We must figure that part out for ourselves! Express the following in scientific notation. a) 21357 2.1357 x 104 b) 0.00374 3.74 x 10-3 c) 238500000 to 4sf 2.385 x 107 d) 238500000 to 7 sf 2.385000 x 107 e) 0.00089700 to 4 sf 8.970 x 10-4 f) 0.00089700 to 2 sf 9.0 x 10-4 Significant Digits in Calculations The answer to a problem cannot have more significance (accuracy) than the quantities used to produce it. Rule 1: Multiplying or Dividing The answer should have the same number of significant figures as the quantity with the fewest significant figures. Problem: Calculate (3.23 x 0.02704)/(250. x 15) to the correct # of s.f. When you have mixed multiplication and division, determine the # of sig figs in each intermediate result as you go along. Do not round any answers until the very end. Problem: Calculate (3.01-1.2)/(3.56 +9.23) to the correct # of sig figs. Problem: Calculate 1.68 x 10-1 / 08.40 x 102 to the correct # of sig figs. Chapter 1 (Part 2) Page 10 of 22 Rule 2: Adding or Subtracting The number of decimal places the answer should equal to the number of decimal places in the number with the fewest decimal places. Problem: Add 3.295 + 10.2 + 0.00001 to the correct # of sig figs. Problem:Subtract 4.2 from 15.723 to the correct # of sig figs. Where do the significant digits that we claim in the previous exercises come from? – Measurements!!! Chapter 1 (Part 2) Page 11 of 22 Accuracy and Precision These two terms are often confused for being synonymous. Accuracy vs Precision Precision The precision of a measurement indicates how well several determinations of the same quantity agree with each other. Accuracy describes how well a measured value agrees with the established correct value. Precise Accurate Both Neither Chapter 1 (Part 2) Page 12 of 22 1.12 Problem Solving: Unit Conversion and Dimensional Analysis Unit Conversions • Information is often not given in the units we need or that we can relate to. Example Problem: if a horse stands 16 hands tall, the average person would want to know the height in inches or feet or meters to better comprehend the information. • The simplest way to carry out calculations involving different units is to use the factor-label method (aka dimensional analysis or unit cancellation). To solve the problem above we would need to know that 1 hand = 4 inches • Units are treated like numbers and can thus be multiplied and divided. • Anything divided by itself = 1 • Anything multiplied by 1 = original value (in new units) • Set up an equation so that all unwanted units cancel. For above problem: SOLUTION Problem : A child is 21.5 inches long at birth. How long is this in centimeters? Chapter 1 (Part 2) Page 13 of 22 What if units are squares or cubes? Problem: Convert 24.5 in2 to m2. Significant Figures for Unit Conversions All English/English conversion factors have unlimited sig figs. All Metric/Metric conversion factors have unlimited sig figs. All English/Metric conversions have limited sf except 1in=2.54cm. Problem: (Metric Conversion) How many centigrams are in a kilogram? If you are asked to convert from one prefix to another, remove the first, then add the second. Chapter 1 (Part 2) Page 14 of 22 1.13 Temperature, Heat, & Energy Energy The capacity to do work or supply heat. Temperature The measure of the average kinetic energy (energy of motion) of the particles. Simple definition: A measure of how hot or cold an object is. Temperature – a measure of the heat energy. Fahrenheit Temperature Scale Defined by German scientist Daniel Gabriel Fahrenheit in late 1600s or early 1700s. Defined by freezing temperature of saturated salt solution (intended to be 0F) and temperature of the human body (intended to be 100F, turned out to be 98.6F) Current markers are freezing temperature of water (___) and boiling point of water (____) Currently in use primarily only in the United States Celsius Temperature Scale Suggested by Swedish astronomer Anders Celsius in the mid 1700s. Sometimes referred to as the _________. Chapter 1 (Part 2) Page 15 of 22 Defined by freezing temperature of water ( _C ) and boiling point of water ( C) Kelvin Temperature Scale Both Fahrenheit and Celsius temperature scales require the use of negative numbers. However, there is a limit to how low temperature can go. This was discovered through hundreds of experiments. William Thompson (a.k.a. Lord Kelvin ) suggested in mid to late 1800s a scale that does not use negative numbers. The Kelvin scale assigns a value of 0 K to the coldest possible temperature, __________, which is equal to 273.15 C It uses the same degree size as the Celsius scale, but starts at absolute zero, or -273.15C. (Expressed simply as K not K) Temperature Conversions Conversion Factors 9 F * C 32 5 K = C + 273.15 C 5 ( F 32 F ) 9 C = K – 273.15 Problem: If it is 20F outside, what is the temp. in C? Problem: If it is 75F outside, what is the temp. in K? Chapter 1 (Part 2) Page 16 of 22 Energy and Heat Energy Two basic forms of Energy Kinetic Energy: The energy of motion. Potential Energy: Stored Energy Bouncing ball converts energy between KE & PE Heat Energy (and heat) are measured in units of Joules (J) in SI units or calories (cal) in metric. 1 cal = 4.184 J 1 cal = the heat required to raise 1 gram of water 1C. 1000 cal = 1 kcal = 1 Cal This is the dietary calorie with a capital C! Specific Heat (c) is the amount of heat required to raise the temperature of 1 gram of substance 1 degree Celsius. Chapter 1 (Part 2) Page 17 of 22 Substance J/g.°C Gold 0.126 Copper 0.386 Cast Iron 0.460 Steel 0.490 Granite 0.790 Glass 0.840 Aluminum 0.900 Water 4.186 How much energy in Joules is needed to raise the temperature of 75.0 g aluminum bar from refrigerator temperature 3.0 °C to 13.0°C? In calories? How much heat (calories) is removed from a 12 oz diet Coke is removed when it is cooled from room temperature (25°C) to 3°C? (Diet coke has the same specific heat as water = = ). 4.186 [[HINT : 1 oz = 28.35 g]] Chapter 1 (Part 2) Page 18 of 22 1.14 Density and Specific Gravity Density Density is the ratio of the mass of an object to its volume. Mass Density = -----------Volume Density is a characteristic property of a substance. It usually has units of g/cm3 or g/mL. Density is stated at a given temperature. Density of Water = 1.00 g/ml at 4 degrees C. (It’s maximum density.) Substances with lower densities will float on ones with higher densities. LP#1. What is the density of 5.00 mL of serum if it has a mass of 5.23 grams? What would the mass of 1.00 liters of this serum be? Chapter 1 (Part 2) Page 19 of 22 Measuring density of Solids - Measure the mass of the solid before submerging it in water to determine its volume. Mass = - Volume displacement is the volume of a solid calculated from the volume of water displaced when it is submerged. - To get the density, divide the mass by the volume. Specific Gravity Specific Gravity is the density of a substance compared to a reference substance Density of Substance Specific Gravity = ---------------------------------------------------------------------Density of Reference Substance (typically water at 4C) It is a ratio with no units. At normal temperatures, the specific gravity of a substance is numerically equal to its density, but has no units. Chapter 1 (Part 2) Page 20 of 22 The specific gravity of a liquid can be measured using an instrument called a hydrometer. Hydrometers contain a weighted bulb at the end of a calibrated glass tube. The depth to which the hydrometer sinks in the fluid indicates the fluid's specific gravity. The lower the hydrometer sinks, the lower the specific gravity. Urinometers (a specialized hydrometer) are used to measure dissolved solids in urine. Urinometers can help identify dehydration. Obesity and Body Fat Show Galileo thermometer picture to class. Body Mass Index weight ( kg ) weight (lb) BMI x 270 2 height (in) 2 height m BMI of 25 -30 is considered overweight. BMI of 30 or above is considered obese. By these standards, approximately 61% of the U.S. population is overweight. The lowest death risk from any cause, including cancer and heart attack was for BMI 22-24. By BMI of 29, the risk doubles! (McMurry 7th Ed.) Chapter 1 (Part 2) Page 21 of 22 Practice Problem for Dimensional Analysis using BMI. Based on the BMI scale, is a 5’10’ man who weighs 220 lb obese? Mass = Height = BMI = Chapter 1 (Part 2) Page 22 of 22