Retinal Progenitor Cell Xenografts to the Pig Retina
advertisement

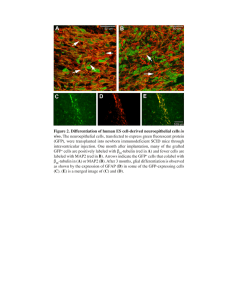
LABORATORY SCIENCES Retinal Progenitor Cell Xenografts to the Pig Retina Morphologic Integration and Cytochemical Differentiation Karin Warfvinge, PhD; Jens F. Kiilgaard, MD, PhD; Erin B. Lavik, DSc; Erik Scherfig, MD; Robert Langer, PhD; Henry J. Klassen, MD, PhD; Michael J. Young, PhD Objective:: To investigate the survival, integration, and differentiation of mouse retinal progenitor cells after transplantation to the subretinal space of adult pigs. Methods:: Green fluorescent protein–positive (GFP⫹) murine retinal progenitor cells were transplanted subretinally as single cells, spheres, or biodegradable polymerprogenitor composites into 24 nonimmunosuppressed adult pigs. Of these, 14 pigs received laser lesions (n=11) or outer retinal scraping injury (n = 3). Recipients were killed at 30 minutes to 5 weeks after grafting. Results: The GFP⫹ murine retinal progenitor cells survived well for up to 14 days after transplantation to the pig retina. After 5 weeks, fewer GFP⫹ cells were found. In the pigs that received laser treatment before grafting of cell suspension, GFP⫹ cells integrated into the retinal pigment epithelium and all layers of the retina. The GFP⫹ cells exhibited morphologic evidence of differentiation into mature retinal neurons, although evaluation of marker expression found only nestin and glial fibrillary acidic protein colocalization. In noninjured pigs, cells mainly integrated into the retinal pigment epithelium. In pigs that received composites, cells appeared to Author Affiliations: Department of Ophthalmology, Lund University Hospital, Lund, Sweden (Dr Warfvinge); Eye Department, Rigshospitalet and Eye Pathology Institute, Copenhagen University, Copenhagen, Denmark (Drs Kiilgaard and Scherfig); Department of Electrical Engineering and Computer Science, Massachusetts Institute of Technology, Cambridge, Mass (Drs Lavik and Langer); Stem Cell Research, Children’s Hospital of Orange County, Orange, Calif (Dr Klassen); and Schepens Eye Research Institute, Department of Ophthalmology, Harvard Medical School, Boston, Mass (Dr Young). T mature and extended processes through pores in the polymer matrix. Conclusions: Retinal progenitor cell xenografts survive for a sufficiently long period to integrate into areas of injury and exhibit morphologic differentiation. By 5 weeks, survival diminishes. Biodegradable polymers may be useful for transplanting retinal progenitor cells in a structurally organized manner. Clinical Relevance: Central nervous system (CNS) diseases may cause long-term disabilities. Substantial tissue destruction can be sustained by the complex structures of the brain, spinal cord, or retina without loss of life, yet the lack of effective CNS regeneration frequently results in disruption of activities of daily living and marked degradation in quality of life. It has become clear that an enormous potential for repair is present within the mammalian CNS. The challenge is to harness this potential to treat disease. Transplantation of neuronal tissue to the CNS represents a promising, albeit challenging, approach to the replacement of neurons lost owing to injury or disease. Arch Ophthalmol. 2005;123:1385-1393 HE MAJOR DISEASES OF THE retina, including retinitis pigmentosa, age-related macular degeneration, and diabetic retinopathy, are quite heterogeneous with respect to etiology, pathology, and demographics, yet have in common the unifying feature of neuronal loss. As in the brain and spinal cord, loss of neurons in the retina is permanent, and there are at present no restorative treatments available for these conditions. One approach to neuronal repopulation that has been actively investigated is the transplantation of embryonic or fetal retinal tissue. Achieving widespread functional integration of transplanted retinal tissue in the diseased retina has proved to be elusive1; however, neural progenitor cells have recently demonstrated considerable potential as an (REPRINTED) ARCH OPHTHALMOL / VOL 123, OCT 2005 1385 alternative to fetal tissue in the setting of transplantation. In rodents, a variety of neural precursor cells have been transplanted to the intact retina of normal neonatal and adult animals, to the degenerating retina, and to the injured retina, with markedly varying results in terms of integration, migration, and differentiation.2,3 In all studies of this type, an important consideration that has emerged is the need to distinguish donor cells from host tissue after transplantation. The use of soluble proteins such as green fluorescent protein (GFP) as a label confers the advantage of demonstrating the detailed configuration of the grafted cells. Recently, isolation and amplification of stem cells from the retina of neonatal mice expressing the enhanced version of the GFP transgene were described.4 After ex- WWW.ARCHOPHTHALMOL.COM Downloaded from www.archophthalmol.com at Danmarks Natur og Laegevidenskabelige Bibliotek, on February 13, 2006 ©2005 American Medical Association. All rights reserved. pansion in vitro, these cells were transplanted to the retina of mice with retinal degeneration. The grafted cells survived, maintained GFP expression, migrated, and showed evidence of integration within the host retina. Herein we describe the transplantation of these GFP mouse progenitor cells to the subretinal space of adult pigs, as cell suspension, spheres, or a composite graft incorporating a biodegradable polymer. The pig is of interest as a recipient because the anatomy, size, and vasculature of the porcine eye more closely approximate the human eye than does that of the rodent, and the techniques and instrumentation routinely used in the clinic are directly applicable to the pig model. Moreover, the porcine retina has an area centralis that contains a large number of cones. METHODS PROGENITOR CELLS Retinal progenitor cells (RPCs) were isolated from pooled retinas of postnatal day 1 GFP transgenic C57Bl/6 mice (a kind gift of M. Okabe, PhD, University of Osaka, Osaka, Japan). Retinas were dissected free from the posterior eyecup, and the ciliary marginal zone and optic nerve head were removed under microscopic visualization. Pooled retinal tissue was finely minced and digested with 0.1% type 1 collagenase (Sigma-Aldrich Corp, St Louis, Mo) for 20 minutes. The supernatant containing liberated cells was forced through a 100-µm mesh strainer, centrifuged, and seeded into culture vessels in basal medium (Neurobasal; GIBCO BRL Life Technologies, Rockville, Md) supplemented with 2-mmol/L L-glutamine, 100-µg/mL penicillin-streptomycin, 20-ng/mL epidermal growth factor (Promega, Madison, Wis), and B-27 neural supplement (GIBCO BRL Life Technologies). This cycle was repeated until all retinal tissue was digested. Cells were re-fed on alternating days. Within 2 to 3 weeks, RPCs were visible as nonadherent spheres and continued to expand in the presence of epidermal growth factor. Cultures were split 1:5 every 7 to 10 days. Although capable of expansion through more than 60 passages, cells of less than 20 passages were used in this study. POLYMER SCAFFOLDS The biodegradable polymer was formed into highly porous scaffolds. These were fabricated from blends of poly(lactic-coglycolic acid) and poly(L-lactic acid) by means of a freezedrying technique that led to pores being oriented normal to the plane of the scaffold. The RPCs were seeded onto the polymers and cultured under expansion conditions (20-ng/mL epidermal growth factor) for 14 days before transplantation. The fabrication of the biodegradable polymer scaffolds has recently been described by Lavik et al.5 ANIMALS AND SURGERY Twenty-four female domestic pigs of the Danish Landrace breed (age, 4 months; weight, approximately 30 kg) were used in the experiments. All animals were preanesthetized with intramuscular injections of 15 mg of midazolam (DormicumA; Roche, Hvidovre, Denmark) and a combination of zolazepam hydrochloride, 11.9 mg/mL, and tiletamine hydrochloride, 11.9 mg/mL (Zoletil 50 Vet; Virbac SA, Carros, France) mixed with xylazine hydrochloride (12.38 mg/mL; Intervet, Skovlunde, Denmark), ketamine hydrochloride (14.29 mg/mL; Intervet), and methadone (2.38 mg/mL; Nycomed, Roskilde, Denmark). The (REPRINTED) ARCH OPHTHALMOL / VOL 123, OCT 2005 1386 pigs underwent endotracheal intubation and were artificially ventilated and anesthetized with 2% to 3% isoflurane (Abbott, Solna, Sweden) in combination with oxygen. The stroke volume (300 mL/stroke) and respiratory frequency (12/min) were kept constant throughout the surgery. The left pupil was dilated with an eyedrop combination of 0.4% benoxinate hydrochloride (oxybuprocaine; SAD, Copenhagen, Denmark), 10% phenylephrine hydrochloride (metaoxedrine chloride; SAD), 0.5% tropicamide (Mydriacyl; Alcon, Heinaut, Belgium), 1% atropine sulfate (SAD), and 5% povidone-iodine (SAD). At surgery, the central and posterior vitreous was removed together with the posterior hyaloid membrane by means of a localized 3-port pars plana vitrectomy. With the goal of promoting integration of grafted cells into the host retina, focal damage was induced in 11 animals via the application of green argon laser burns in a grid pattern to the area centralis. The laser lesions were placed as a triangle with the apex close to the optic disc. The energy of the lesions was chosen from peripheral test lesions to give a uniform, white color of the spot without signs of perforation or necrosis. Thereafter, a retinal bleb was elevated in the same area by the injection of 0.25 to 0.5 mL of 0.9% sodium chloride through a 41-gauge needle. The use of endodiathermy on the detached retina preceded further enlargement of the retinotomy before transplantation. In 3 animals, mechanical scraping of the outer retina was performed before grafting to the area centralis of the polymerprogenitor composite (no laser treatment was given). The murine GFP-positive (GFP⫹) RPCs were injected into the retinal bleb as a single-cell suspension by using a 27-gauge needle (n=12), as spheres of cells by using a 20-gauge needle (n=6), or as a biodegradable polymer-progenitor composite by using fine forceps (n=6). The single-cell suspension and sphere injections contained approximately 2⫻107 cells, while the biodegradable polymer composite graft contained approximately 2⫻106 cells. Immediate reflux of some cells into the vitreous cavity was observed in most animals injected with cell suspension or spheres. A small air bubble was placed in the subretinal bleb under the retinotomy to prevent further reflux after withdrawal of the needle. Chloramphenicol (SAD) was given locally at the end of surgery to prevent infection. The pigs were examined by ophthalmoscopy on a weekly basis. The research protocol was reviewed and approved by the Danish Animal Experiment Inspectorate, and was in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. TISSUE PROCESSING Eyes were enucleated with the animal under anesthesia at 30 minutes (suspension, n=1), 1 week (suspension, n=3; spheres, n=2; polymer, n=2), 2 weeks (suspension, n=2; spheres, n=2; polymer, n=2), and 5 weeks (suspension, n=6; spheres, n=2; polymer, n=2) after transplantation. After enucleation, pigs were killed by intravenous injection of 2 to 4 g of pentobarbital sodium (200 mg/mL; KVL, Copenhagen). Globes were placed in 4% paraformaldehyde for 10 to 20 minutes. The anterior segment and the lens were then removed and the posterior segment was postfixed for 2 hours in 4% paraformaldehyde, with subsequent rinsing in increasing concentrations of sucrose containing Sörensen phosphate buffer. A horizontal cut was made that extended from the temporal retinal margin to 2 to 3 mm nasal to the optic disc, thus comprising the temporal ciliary margin, the area centralis, and the optic disc. The tissues were embedded in a gelatin medium and serially sectioned at 12 µm on a cryostat. During the sectioning process, every 15th section was examined by epifluorescence microscopy for GFP⫹ cells and every 10th slide was stained with hematoxylin-eosin. WWW.ARCHOPHTHALMOL.COM Downloaded from www.archophthalmol.com at Danmarks Natur og Laegevidenskabelige Bibliotek, on February 13, 2006 ©2005 American Medical Association. All rights reserved. Table 1. Primary Antibodies Antigen Species Description GFP Chicken polyclonal PCNA Mouse monoclonal Vimentin Mouse monoclonal Green fluorescent protein Proliferating cell nuclear antigen Vimentin Used for Detection of Dilution Grafted cells 1:5000 Chemicon, Temecula, Calif GFAP Rabbit polyclonal Proliferative cells 1:500 1:10 1:100 DAKO 1:100 1:200 Mouse monoclonal Microtubule-associated protein 2 RPE-specific protein 65 Müller cells and astrocytes Müller cells and astrocytes Immature cells and activated glial cells Neural precursor cells Santa Cruz Biotechnology, Santa Cruz, Calif DAKO, Copenhagen, Denmark Nestin Mouse monoclonal RPE 1:200 Cytokeratin Recoverin Mouse monoclonal Rabbit polyclonal Cytokeratin Recoverin RPE Cones 1:100 1:10 000 Transducin Rho 4D2 Rabbit polyclonal Mouse monoclonal G protein G-␥-c subunit N-terminal of rhodopsin Cones Rods 1:1000 1:100 Parvalbumin Mouse monoclonal Parvalbumin 1:1000 Calbindin PKC Mouse monoclonal Mouse monoclonal Calbindin Protein kinase C Bipolar and amacrine cells Horizontal cells Bipolar cells BD Biosciences Pharmingen, Heidelberg, Germany Sigma-Aldrich Corp, St Louis, Mo Gift of D. Thompson, PhD, Ann Arbor, Mich DAKO Gift of A. Dizhoor, PhD, Detroit, Mich CytoSignal, San Diego, Calif Gift of R. S. Molday, PhD, Vancouver, British Columbia Sigma-Aldrich Corp MAP-2 Mouse monoclonal RPE65 Glial fibrillary acidic protein Intermediate filaments 1:200 1:1000 Source Sigma-Aldrich Corp Nordic Biosite AB, Täby, Sweden Abbreviation: RPE, retinal pigment epithelium. IMMUNOHISTOCHEMISTRY The retinal sections were exposed to primary antisera (Table 1) in a moist chamber for 16 to 18 hours, at 4°C, followed by rinsing in 0.1M phosphate-buffered saline with 0.25% Triton X-100. Sections were then incubated with secondary Texas red– conjugated antibodies (1:200; Jackson Immunoresearch, West Grove, Pa) for 1 to 2 hours at room temperature in the dark. Normal eyes, processed in parallel, were used as controls. In addition, negative controls with omission of the primary antisera were performed. The specimens were examined with an epifluorescence microscope. Colocalization of Texas red–labeled primary antibodies and GFP⫹ cells was assessed by superimposition of separate digital images of each fluorochrome. Sections were frequently stained with chicken anti-GFP, either to examine the quality of the GFP expression or to disclose possible down-regulated grafted cells. The endogenous GFP expression was always high, and enhancing with anti-GFP resulted in blurring of the cell boundaries. In the pigs with few or no surviving cells, the anti-GFP did not disclose any additional cells; therefore, these results will not be further discussed. associated protein 2 (MAP-2) were expressed at some point, but not vimentin, retinal pigment epithelium (RPE)–specific protein 65, cytokeratin, recoverin, transducin, Rho 4D, parvalbumin, calbindin, or protein kinase C. Specific data concerning pretreatment conditions and GFP⫹ cell integration and migration are summarized in Table 2 and Table 3. POSTTREATMENT REACTIONS IN THE HOST RETINA In all pigs killed 1 to 2 weeks after grafting, the host retina showed up-regulation of both GFAP and vimentin. This up-regulation corresponded to the location of the subretinal bleb induced at the time of transplantation and thus the area of transient focal detachment. Eleven of the 24 animals were pretreated with laser burns in an effort to enhance donor cell integration. Histologically, areas of the retina with laser burns exhibited decreased thickness, laminar disorganization, and occasional intraretinal RPE cells. RESULTS Retinal progenitor cells derived from postnatal day 1 GFP transgenic mice were transplanted to the subretinal space of 24 mature pigs, as single-cell suspension, as spheres, or as a biodegradable polymer-progenitor composite. After death of the animal at various time points ranging from 30 minutes to 5 weeks after transplantation, surviving GFP⫹ cells were found at or near the transplantation site in 16 (67%) of the 24 animals. A vast number of markers (Table 1) were examined before and after grafting. Only proliferating cell nuclear antigen (PCNA), nestin, glial fibrillary acidic protein (GFAP), and microtubule(REPRINTED) ARCH OPHTHALMOL / VOL 123, OCT 2005 1387 GFP TRANSGENIC MURINE RPCS: BEFORE GRAFTING Cells prepared as single-cell dissociates, as well as cells grown as spheres, displayed immunoreactivity to the markers PCNA, nestin, GFAP, and MAP-2. No baseline reactivity was detected with regard to the other antibodies tested, as listed in Table 1. Mouse RPCs seeded onto a scaffold were found to be situated largely at the surface of the polymer, were rounded, and maintained immunoreactivity to PCNA, nestin, GFAP, and MAP-2 (Figure 1). WWW.ARCHOPHTHALMOL.COM Downloaded from www.archophthalmol.com at Danmarks Natur og Laegevidenskabelige Bibliotek, on February 13, 2006 ©2005 American Medical Association. All rights reserved. Table 2. Method of GFPⴙ Cell Delivery, Survival Times, Pigs Grafted, Surviving Cells, and Retinal Damage Before Transplantation Cell Deliverables, Pig No./No. of Surviving Cells (Pretreatment) Survival Time Suspension 30 min 1 wk 64/⫹⫹⫹ (None) 55/⫹⫹⫹ (None) 73/⫹⫹⫹ (Laser) 74/⫹⫹⫹ (Laser) 56/⫹⫹⫹ (None) 75/⫹⫹⫹ (Laser) 57/⫹ (None) 76/− (Laser) 107/− (Laser) 108/− (Laser) 109/⫹ (Laser) 110/− (Laser) 2 wk 5 wk Spheres Polymer-Progenitor Composite 54/− (None) 77/⫹⫹ (Laser) 58/⫹⫹⫹ (None) 80/⫹⫹⫹ (Scraped) 61/⫹⫹ (None) 81/⫹⫹ (Laser) 62/⫹ (None) 79/⫹ (Laser) 63/− (None) 78/⫹⫹⫹ (Scraped) 59/− (None) 82/− (Scraped) Abbreviations: GFP⫹, green fluorescent protein–positive; −, no cells; ⫹, few cells; ⫹⫹, moderate number of cells; ⫹⫹⫹, many cells. Table 3. Pigs With Surviving Cells and Integration and Migration Into the Retina of GFPⴙ Cells After Grafting Integration Suspension Spheres Polymer-progenitor composite Pig No. Migration Into the Retina RPE or Retina Subretinal Space or Vitreous 64 55 73 74 56 75 57 109 77 61 81 62 79 58 80 78 − − ⫹⫹⫹ ⫹⫹⫹ ⫹ ⫹⫹⫹ − − − − − − − − − − None RPE RPE, retina RPE, retina RPE, retina RPE, retina None RPE RPE None None None None None None None Subretinal space None Vitreous Subretinal space None Vitreous Subretinal space None Subretinal space, vitreous Subretinal space, vitreous Subretinal space, vitreous Subretinal space Subretinal space Cells only in composite Cells only in composite Cells only in composite Abbreviations: GFP⫹, green fluorescent protein–positive; RPE, retinal pigment epithelium; −, none; ⫹, few cells found in a restricted area of the retina; ⫹⫹⫹, many cells in all layers of the retina. CELL SUSPENSION: AFTER GRAFTING To validate the surgical method used for subretinal transplantation, 1 pig that had received RPCs as a single-cell suspension was killed 30 minutes after grafting. At this time, a large retinal detachment was evident (Figure 2A). Rounded GFP⫹ cells were found floating freely in the subretinal space or attached to the RPE or photoreceptor outer segments (Figure 2B and C). The GFP⫹ cells coexpressed nestin, and some were in a proliferative stage, as shown by PCNA immunostaining. Some GFP⫹ cells coexpressed GFAP or MAP-2. Three pigs were killed 1 week after transplantation, 2 of which had been treated with retinal burns at the time of surgery, and surviving RPCs were found in all 3 animals. In the laser-treated pigs, GFP⫹ cells integrated into the RPE (Figure 3A and D) and all layers of the retina (Figure 3B-E) and were also found in or close to the nerve fiber layer (Figure 3F). The cells (REPRINTED) ARCH OPHTHALMOL / VOL 123, OCT 2005 1388 had matured and exhibited a range of phenotypes, the majority appearing morphologically neuronlike. In the untreated pig, GFP⫹ cells were mainly found in the RPE layer, although occasional GFP⫹ fibers were seen in the retina as well (data not shown). Immunohistochemistry showed expression of nestin (Figure 3F) and GFAP (Figure 3G) by donor cells, but not vimentin (Figure 3H). Autofluorescence throughout the entire retina was a common finding in all the laser-treated animals (Figure 3A-C). Two pigs were killed 2 weeks after transplantation, 1 of which had been treated with laser burns, and surviving donor cells were found in both animals. In the untreated pig, GFP⫹ cells had integrated into the RPE layer, although, in contrast to host RPE cells, the donor cells did not express the RPE-specific markers RPE65 or cytokeratin ( Figure 4 A and B). In the laser-treated animal, GFP⫹ cells were found in the retina at or near the laser wounds. Despite the morWWW.ARCHOPHTHALMOL.COM Downloaded from www.archophthalmol.com at Danmarks Natur og Laegevidenskabelige Bibliotek, on February 13, 2006 ©2005 American Medical Association. All rights reserved. HTX-Eosin B GFP C PCNA E GFAP F MAP-2 Polymer A D Nestin Figure 1. Polymer-progenitor composite cultured for 14 days (scale bars, 90 µm). A, Hematoxylin (HTX)-eosin staining of the composite shows blue nuclei of the mouse cells covering the surface of the polymer. The vertical line on the left side indicates the thickness of the polymer. B, Visualization of the composite by fluorescence microscopy shows green fluorescent protein (GFP)–positive (GFP⫹) profiles on the polymer surface. C, Immunohistochemistry of the composite using antibodies against the proliferating cell nuclear antigen (PCNA). Double-labeling of PCNA (red) and GFP shows that most cells are in a proliferative stage (yellow-orange). D-F, Subpopulations of GFP⫹ cells also express nestin (D), as well as glial fibrillary acidic protein (GFAP) (E) and microtubule-associated protein 2 (MAP-2) (F). GFP A B C RPE ONL ONL Figure 2. Retinal progenitor cell suspension 30 minutes after grafting to the pig eye. A, A large area of retinal detachment is evident (hematoxylin-eosin, original magnification ⫻8). B, Under the detachment, cells are found floating within the subretinal space or adhering to the retinal photoreceptors (ONL indicates outer nuclear layer) (hematoxylin-eosin; scale bar, 45 µm). C, Fluorescent microscopy shows that the grafted cells express green fluorescent protein and in some cases adhere to the photoreceptors (arrowheads) or retinal pigment epithelium (RPE) (arrows) (scale bar, 45 µm). phologic evidence of donor cell differentiation, immunohistochemical studies were negative for markers of neuronal phenotype. Six animals were killed 5 weeks after transplantation, 5 with laser treatment and 1 without. Survival was markedly decreased at this point, with surviving cells being found in only 2 pigs (1 laser treated, 1 untreated). The surviving cells were very few and immunohistochemical analysis was not undertaken. SPHERES: AFTER GRAFTING Two animals injected with RPCs as spherical aggregates were killed 1 week after grafting, 1 of which had been treated with laser and 1 of which was untreated. The treated pig displayed surviving GFP⫹ cells, and these were located in the subretinal space and the vitreous (REPRINTED) ARCH OPHTHALMOL / VOL 123, OCT 2005 1389 (Figure 5A and B). These cells were found either as spheres or as single cells, a few of which had extended processes at this point. A small number of cells had also integrated into the retina. As seen in the pigs grafted with cell suspension, only nestin and GFAP colocalized with the GFP⫹ profiles (Figure 5C). Two animals were killed 2 weeks after grafting, 1 laser treated and 1 untreated. In the laser-treated pig, single cells or layers of cells were seen covering the inner (vitreal) surface of the retina. These cells were immunoreactive for nestin and GFAP. In the untreated pig, spheres of GFP⫹ cells were found in the vitreous. These cells were also nestin and GFAP positive. Two pigs were killed 5 weeks after grafting, 1 laser treated and 1 untreated. Both animals displayed foci where a few GFP⫹ cells had been incorporated into the RPE. Immunohistochemical analysis was not carried out. WWW.ARCHOPHTHALMOL.COM Downloaded from www.archophthalmol.com at Danmarks Natur og Laegevidenskabelige Bibliotek, on February 13, 2006 ©2005 American Medical Association. All rights reserved. A RPE B RPE Subretinal Space C ONL INL IPL D RPE E INL IPL NF F RPE Nestin G RPE GFAP H RPE Vimentin Figure 3. Integration and differentiation patterns 1 week after grafting as cell suspension (scale bars, 60 µm). Panels F through H show immunolabeling with different antibodies (scale bars, 45 µm). A, Integration of green fluorescent protein (GFP)–positive (GFP⫹) donor cells into the retinal pigment epithelium (RPE). B, In this image retinal progenitor cells are not integrated into the RPE, but form a layer covering the photoreceptors. Green fluorescent protein–positive donor cells are also found in the outer retinal layers, sending radially oriented processes into the inner retina (arrows). C, All retinal layers harbor GFP⫹ cells in this section. Processes are found reaching both radially and horizontally. Autofluorescent bodies (yellow) are also seen within the retina. ONL indicates outer nuclear layer; INL, inner nuclear layer; and IPL, inner plexiform layer. D, In this section, murine retinal progenitor cells have integrated into the RPE and retina. Radially oriented GFP⫹ fibers are present (arrows). In the middle of the image a donor cell, probably situated in the INL, sends processes into the ONL. E, This image shows GFP⫹ cells in the nerve fiber layer (NF), but also a well-developed cell (arrow) in the INL. F, A layer of GFP⫹ donor cells that coexpress nestin is found covering the photoreceptors (arrowheads). Some cells send processes into the retina (arrow). G, Up-regulation of glial fibrillary acidic protein (GFAP) is found in the host retina. This up-regulation corresponded to the location of the subretinal bleb induced at the time of transplantation and thus the area of transient focal detachment. Some of the GFAP-positive cells coexpress GFP, consistent with donor cells (arrowheads). H, Vimentin is also up-regulated in the host corresponding to the area of local detachment, but no coexpression of vimentin and GFP was found. BIODEGRADABLE POLYMER-PROGENITOR COMPOSITE Two animals transplanted with biodegradable polymerprogenitor composite were killed 1 week after grafting, 1 that had been treated with subretinal scraping and 1 untreated. Surviving GFP⫹ cells were found in both pigs. Grafted cells were generally confined to the polymer structure and exhibited neuronallike and gliallike phenotypes, often with long slender processes extending across the width of the scaffold (Figure 6). Again, some of the GFP⫹ cells colabeled with nestin or GFAP. (REPRINTED) ARCH OPHTHALMOL / VOL 123, OCT 2005 1390 Two weeks after transplantation, 2 pigs grafted with the polymer-progenitor composite were killed; 1 had received subretinal scraping before transplantation and 1 was untreated. The treated animal contained GFP⫹ cells that exhibited both gliallike and neuronallike morphologic features, as seen in the 1-week pigs. In this case the retina was severely disrupted, likely related to complications at the time of surgery, and further analysis was not performed. Two polymer-grafted animals were killed 5 weeks after grafting. In both cases the scaffolds showed clear signs of biodegradation; however, no surviving cells were found in these specimens. WWW.ARCHOPHTHALMOL.COM Downloaded from www.archophthalmol.com at Danmarks Natur og Laegevidenskabelige Bibliotek, on February 13, 2006 ©2005 American Medical Association. All rights reserved. COMMENT This study is the first, to our knowledge, to demonstrate morphologic integration of transplanted progenitor cells into the neural retina and RPE of a large mammal. This integration occurred in developmentally mature hosts (3 months) during a relatively short period (within 1 week) and despite substantial genetic disparity between donor (mouse) and recipient (pig). This study also demonstrates the feasibility of using biodegradable polymerprogenitor composite for cell delivery in a large, humanlike eye, although no cells escaped the biopolymer scaffold after transplantation into the pig eye. All findings were obtained in the absence of active immunosuppression, a fact that has direct bearing on the decrease in donor cell survival seen at 5 weeks. Preliminary data indicate that a substantial host immune response, initiated by preformed antibodies and directed against the foreign donor cells, is responsible for this decreased survival.6 Previous reports on the transplantation of central nervous system progenitor cells to the retina include studies in the rat,7-10 mouse,4 and Brazilian opossum.11 The work in the pig presented herein extends the existing literature to a large animal model where the biological and surgical challenges more closely approximate those faced in the human retina. The surgical approach used in this study, namely, pars plana vitrectomy followed by creation of a retinotomy and the raising of a focal retinal bleb, proved to be a reliable method for accessing the subretinal space. This was demonstrated by results from the pig killed 30 minutes after grafting, as well as the results from later times. All retinal blebs resolved and the retina was reattached by the end of the first week. In response to the transplant procedure, the host retina up-regulated GFAP and vimentin, consistent with an activation of Müller cells and astrocytes; however, there was no further evidence of the remodeling events that can occur in the retina as a complication of retinal detachment.2,12-14 Specifically, gliosis and subretinal fibrosis were not observed at later points. Of the 3 methods of delivery used in the present study, dissociated cell suspension was the simplest way to deliver these cells and resulted in maximal intraretinal migration. In addition, a drawback to the sphere delivery method was the necessity to extend the retinotomy to accommodate the larger-gauge cannula needed for delivery. This resulted in an increased tendency toward reflux of GFP⫹ spheres into the vitreous cavity. Consistent with this observation, the histologic data from animals with surviving grafts showed GFP⫹ cells present in the vitreous of 3 (60%) of 5 animals when grafted as spheres, compared with 2 (25%) of 8 for suspension and 0 (0%) of 3 for polymer-progenitor composite. Cells grafted on the biodegradable polymer were invariably found in the subretinal space (100% [3/3]) and remained within the polymer scaffold for the duration of the study without migration into the host retina or RPE. The mouse and the pig are distantly related species, and therefore the risk of immune rejection is high for grafts between these animals. Furthermore, while there is growing literature exploring the factors underlying the rejec(REPRINTED) ARCH OPHTHALMOL / VOL 123, OCT 2005 1391 A RPE Cytokeratin Subretinal Space ONL INL IPL B RPE65 Figure 4. Immunolabeling of a noninjured retina for the retinal pigment epithelium (RPE) markers cytokeratin (A) and RPE65 (B) (scale bars, 180 µm). No double-labeling of the green fluorescent protein–positive cells integrated into the RPE layer was observed. ONL indicates outer nuclear layer; INL, inner nuclear layer; and IPL, inner plexiform layer. tion of porcine cells transplanted to the mouse,15-17 the risk of rejection is at least as great when the pig, with its more sophisticated immune system, serves as the recipient. Nevertheless, the absence of available porcine retinal progenitors together with a number of mitigating considerations led to the decision to go forward with this study. These included the absence of passenger leukocytes in the grafts, the lack of detectable major histocompatibility complex class I or class II expression by murine central nervous system progenitor cells,18,19 and the relative immune privilege associated with the retina as a host site.20,21 No evidence of migration into the host was observed 30 minutes after transplantation, whereas at the 1-week and later points GFP⫹ cells had been integrated into the RPE and all layers of the neural retina, including the nerve fiber layer. There was variability in the degree of integration between animals, and this appeared to relate to treatment condition. Retinal integration of GFP⫹ cells was observed only in animals that received grafts as a dissociated cell suspension. In addition, injuring the retina with laser burns at the time of transplantation appeared to promote retinal and RPE integration. Further support for this association comes from the observation that integration of GFP⫹ cells was centered on areas of laser injury. This tendency toward wound tropism is in accord with a number of other studies showing that developmental immaturity,8,11 lesions, or genetic dystrophies9,22-24 strongly enhance the migration and integration of grafted progenitor cells. A corollary of this tropism concept is that, in the setting of disease, grafted progenitor cells should home to sites where they are needed and avoid interfering with healthy areas of the retina. Compared with previous studies of progenitor cell integration in the retina, the significance of the current study centers on the fact that the porcine eye is much closer to that of huWWW.ARCHOPHTHALMOL.COM Downloaded from www.archophthalmol.com at Danmarks Natur og Laegevidenskabelige Bibliotek, on February 13, 2006 ©2005 American Medical Association. All rights reserved. RPE RPE ONL INL IPL GCL A B Vitreous C GFAP Figure 5. Retinal progenitor spheres after transplantation to a pig that was laser treated. A, A green fluorescent protein–positive sphere in the vitreous (scale bar, 60 µm). B, Green fluorescent protein–positive cells in the vitreous, in the subretinal space, and a few in the retina. Donor cells were found as either spheres or single cells, a few of which had matured to cells with processes (scale bar, 180 µm). RPE indicates retinal pigment epithelium; ONL, outer nuclear layer; INL, inner nuclear layer; IPL, inner plexiform layer; and GCL, ganglion cell layer. C, As seen in the pigs grafted with cell suspension, green fluorescent protein–positive cells double-labeled with glial fibrillary acidic protein (GFAP) (arrowheads). Also evident in this image is up-regulation of GFAP in the host retina (scale bar, 45 µm). Figure 6. Polymer-progenitor composite within the subretinal space. The dashed line represents the border of the polymer. The green fluorescent protein–positive donor cells have a more differentiated appearance and have extended long slender processes that span the cross section of the polymer (scale bar, 90 µm). mans. The vitreous cavity is much larger than that of rodents, while the retina is thicker and contains many more cones. Thus, the results obtained in this large animal model are of particular relevance in the context of the potential clinical applications. The integration of progenitor cells into the RPE is an interesting finding, although the grafted cells did not express either of the 2 RPE-related genes examined. Many studies have shown that transplanted RPE cells tend to adhere to each other in the subretinal space rather than integrating into the host RPE monolayer or binding extensively to Bruch membrane.25-28 In addition, integration into the RPE layer has not been seen in many previous studies of central nervous system progenitor transplantation to the retina.4,8,9 Integration of progenitor cells into the RPE layer was first described by Warfvinge and coworkers7 by using an immortalized rat neural precursor line in the rat. In the present study, the GFP⫹ mouse RPCs were integrated into the pig RPE layer in a manner reminiscent of the earlier rat study, despite the fact that these cells do not exhibit this behavior when grafted to mice.4 Although many questions remain to be answered, particularly with respect to the long-term functional capabilities of grafted cells, the phenomenon of RPE integration raises the possibility of using progenitor cells in diseases with RPE defects, such as age-related macular degeneration. Apart from the 1 case of RPE integration mentioned already, cells grafted as spheres showed no evidence of mi(REPRINTED) ARCH OPHTHALMOL / VOL 123, OCT 2005 1392 gration into the host tissue. Interestingly, the GFP⫹ cells within the scaffolds survived and extended processes with a radial orientation. On the basis of these results, there is little rationale for transplanting cells as spheres in the context of this model; however, the polymer-progenitor composite grafts may provide a means of transplanting cells to the subretinal space in an organized manner. This may be of interest in situations where high densities of donor cells are required to reconstruct the outer nuclear layer, while a widespread migration into the inner retina is not necessary. In addition, the polymer component of the graft can be modified to provide a vehicle for sustained delivery of a range of therapeutic agents.29,30 In culture, and after transplantation to the retina of mice, RPCs from the GFP mouse can be induced to differentiate into glia and neurons, including presumptive photoreceptors.4 After transplantation of these cells to the pig, a similar yet less clear-cut picture emerges. Morphologically, the cells appeared to differentiate into both glial and neuronal phenotypes. However, marker studies only partially confirmed this impression. Cells prepared as single-cell dissociates, as well as cells grown as spheres, displayed immunoreactivity to the proliferative marker PCNA, the immature cell marker nestin, the glial marker GFAP, and the neuronal marker MAP-2. After grafting, there was evidence of expression of GFAP and, to a limited extent, nestin and MAP-2. However, markers specific for retinal neurons were not seen. This implies that the proliferative capacity has ceased and a downregulation of nestin and MAP-2 has occurred over time. Incomplete expression of markers by progenitor cells after transplantation has been previously reported,8 although the basis of this phenomenon remains to be elucidated. The cells in this case started from a developmentally immature state, as evidenced by widespread nestin expression. The persistence of nestin in GFP⫹ cells at later points suggests that many cells remained developmentally immature after transplantation. Insufficient instructional cues may have been available in the porcine retinal microenvironment to provide adequate phenotypic direction to the grafted cells, thereby resulting in incomplete differentiation. Yet another possibility is phenotypic restriction of the donor cells, either in culture or as an artifact of the transplantation process. DeWWW.ARCHOPHTHALMOL.COM Downloaded from www.archophthalmol.com at Danmarks Natur og Laegevidenskabelige Bibliotek, on February 13, 2006 ©2005 American Medical Association. All rights reserved. spite the unanswered questions, this study shows that sufficient cues are present in the mature retina of the pig to allow for a remarkable degree of cross-species integration, even when progenitor cells from a rodent are used. In conclusion, this study demonstrates that the phenomenon of progenitor cell integration seen in the neural retina and RPE of rodents can be replicated in a large animal model. While the degree of integration and differentiation achieved were somewhat less extensive than those seen in mice, rats, and the Brazilian opossum, many options remain available for further development of the pig model, including the development of an allograft model through the isolation of porcine retinal progenitors,31 together with the possibility of grafting into a porcine model of retinal degeneration such as the rhodopsin, transgenic pig.32 In addition, a major advantage of the pig is that it allows for much more detailed functional analysis than is possible in rodents. Finally, the large eye of the pig provides a convenient proving ground for bioengineering strategies, such as cell delivery on biodegradable polymer scaffolds as well as controlled drug delivery, both of which are likely to play an increasingly important role in retinal reconstruction research. Submitted for Publication: November 2, 2004; final revision received January 26, 2005; accepted February 2, 2005. Correspondence: Karin Warfvinge, Department of Ophthalmology, Lund University Hospital, S-221 84 Lund, Sweden. Financial Disclosure: None. Funding/Support: This study was supported by the Richard D. and Gail Siegal Foundation, New York, NY (Drs Lavik, Langer, and Young); the Crown Princess Margareta’s Committee for the Blind, Stockholm, Sweden (Dr Warfvinge); the Swedish Association of the Visually Impaired, Stockholm (Dr Warfvinge); the Swedish Science Council, Stockholm (Medicine) (Dr Warfvinge); Second ONCE International Award for New Technologies for the Blind, Madrid, Spain (Dr Warfvinge); the Danish Eye Foundation, Copenhagen (Drs Kiilgaard and Scherfig); grant NS44060 from the National Institutes of Health, Bethesda, Md; the CHOC Foundation, Guilds, and Padrinos, Orange, Calif (Dr Klassen); and the Minda de Gunzburg Center for Retinal Transplantation, Schepens Eye Research Institute, Boston, Mass (Dr Young). REFERENCES 1. Gouras P, Tanabe T. Survival and integration of neural retinal transplants in rd mice. Graefes Arch Clin Exp Ophthalmol. 2003;241:403-409. 2. Wojciechowski AB, Englund U, Lundberg C, Warfvinge K. The migratory capacity of brain-derived precursor cells and the host glial response, after subretinal transplantation to normal adult rats. Glia. 2004;47:58-67. 3. Klassen H, Sakaguchi DS, Young MJ. Stem cells and retinal repair. Prog Retin Eye Res. 2004;23:149-181. 4. Shatos MA, Mizumoto K, Mizumoto H, et al. Multipotent stem cells from the brain and retina of green mice. J Reg Med. 2001;2:13-15. 5. Lavik EB, Klassen H, Warfvinge K, Langer R, Young MJ. Fabrication of degradable polymer scaffolds to direct the integration and differentiation of retinal progenitors. Biomaterials. 2005;26:3187-3196. 6. Kiilgaard JF, Scherfig E, Klassen H, Warfvinge K, Prause JU, Young MJ. Transplantation of xenogeneic retinal stem cells to pig subretinal space [abstract]. In- (REPRINTED) ARCH OPHTHALMOL / VOL 123, OCT 2005 1393 vest Ophthalmol Vis Sci. 2003;44:e-abstract 487. 7. Warfvinge K, Kamme C, Englund U, Wictorin K. Retinal integration of grafts of brain-derived precursor cell lines implanted subretinally into adult, normal rats. Exp Neurol. 2001;169:1-12. 8. Takahashi M, Palmer TD, Takahashi J, Gage F. Widespread integration and survival of adult-derived neural progenitor cells in the developing optic retina. Mol Cell Neurosci. 1998;12:340-348. 9. Young MJ, Ray J, Whiteley SJO, Klassen H, Gage FH. Neural differentiation and morphological integration of hippocampal progenitor cells transplanted to the retina of immature and mature dystrophic rats. Mol Cell Neurosci. 2000;16:197-205. 10. Mizumoto H, Mizumoto K, Whiteley SJO, et al. Transplantation of human neural progenitor cells to the vitreous cavity of the Royal College of Surgeons rat. Cell Transplant. 2001;10:223-233. 11. Van Hoffelen SJ, Young MJ, Shatos MA, Sakaguchi DS. Incorporation of murine brain progenitor cells into the developing mammalian retina. Invest Ophthalmol Vis Sci. 2003;44:426-434. 12. Lewis GP, Fisher SK. Müller cell outgrowth after retinal detachment: association with cone photoreceptors. Invest Ophthalmol Vis Sci. 2000;41:1542-1545. 13. Lewis GP, Charteris DG, Sethi CS, et al. The ability of rapid retinal reattachment to stop or reverse the cellular and molecular events initiated by detachment. Invest Ophthalmol Vis Sci. 2002;43:2412-2420. 14. Fisher SK, Lewis GP. Müller cell and neuronal remodeling in retinal detachment and reattachment and their potential consequences for visual recovery: a review and reconsideration of recent data. Vision Res. 2003;43:887-897. 15. Wennberg L, Song Z, Bennet W, et al. Importance of the Gal alpha1-3 Gal antigen in discordant islet xenotransplantation: immunosuppression, which inhibits porcine islet xenograft rejection in ordinary mice, is equally effective in Galknockout mice. Transplantation. 2004;77:1275-1280. 16. Costa C, Pizzolato MC, Shen Y, Wang Y, Fodor WL. CD86 blockade in genetically modified porcine cells delays xenograft rejection by inhibiting T-cell and NK-cell activation. Cell Transplant. 2004;13:75-87. 17. McKenzie IF, Li YQ, Xing PX, et al. CD46 protects pig islets from antibody but not cell-mediated destruction in the mouse. Xenotransplantation. 2003;10: 615-621. 18. Klassen H, Schwartz MR, Bailey AH, Young MJ. Surface markers expressed by multipotent human and mouse neural progenitor cells include tetraspanins and non-protein epitopes. Neurosci Lett. 2001;312:180-182. 19. Hori J, Ng TF, Shatos M, et al. Neural progenitor cells lack immunogenicity and resist destruction as allografts. Stem Cells. 2003;21:405-416. 20. Streilein JW. Ocular immune privilege and the Faustian dilemma: the Proctor Lecture. Invest Ophthalmol Vis Sci. 1996;37:1940-1950. 21. Streilein JW, Ksander BR, Taylor AW. Immune deviation in relation to ocular immune privilege. J Immunol. 1997;158:3557-3560. 22. Brustle O, Jones KN, Learish RD, et al. Embryonic stem cell-derived glial precursors: a source of myelinating transplants. Science. 1999;285:754-756. 23. Aboody KS, Brown A, Rainov NG, et al. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci U S A. 2000;97:12846-12851. 24. Otani A, Kinder K, Ewalt K, et al. Bone marrow–derived stem cells target retinal astrocytes and can promote or inhibit retinal angiogenesis. Nat Med. 2002; 8:1004-1010. 25. Algvere PV, Berglin L, Gouras P, Sheng Y, Kopp ED. Transplantation of RPE in age-related macular degeneration: observations in disciform lesions and dry RPE atrophy. Graefes Arch Clin Exp Ophthalmol. 1997;235:149-158. 26. Del Priore LV, Tezel TH, Kaplan HJ. Survival of allogeneic porcine retinal pigment epithelial sheets after subretinal transplantation. Invest Ophthalmol Vis Sci. 2004;45:985-992. 27. Wang H, Yagi F, Cheewatrakoolpong N, Sugino IK, Zarbin MA. Short-term study of retinal pigment epithelium sheet transplants onto Bruch’s membrane. Exp Eye Res. 2004;78:53-65. 28. Klassen H, Whiteley SJ, Young MJ, Lund RD. Graft location affects functional rescue following RPE cell transplantation in the RCS rat. Exp Neurol. 2001; 169:114-121. 29. Langer R, Brem H, Tapper D. Biocompatibility of polymeric delivery systems for macromolecules. J Biomed Mater Res. 1981;15:267-277. 30. Murray J, Brown L, Langer R. Controlled release of microquantities of macromolecules. Cancer Drug Deliv. 1984;1:119-123. 31. Shatos MA, Klassen H, Scherfig E, et al. Isolation, characterization and expansion of porcine retinal progenitor cells [abstract]. Invest Ophthalmol Vis Sci. 2003; 44:e-abstract 1694. 32. Petters RM, Alexander CA, Wells KD, et al. Genetically engineered large animal model for studying cone photoreceptor survival and degeneration in retinitis pigmentosa. Nat Biotechnol. 1997;15:965-970. WWW.ARCHOPHTHALMOL.COM Downloaded from www.archophthalmol.com at Danmarks Natur og Laegevidenskabelige Bibliotek, on February 13, 2006 ©2005 American Medical Association. All rights reserved.