LIGHT BEHAVES LIKE WAVE - DIFRACTION EXPERIMENT and
advertisement

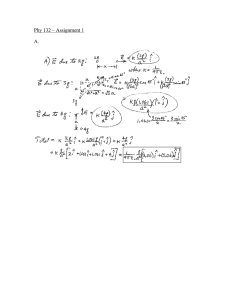
LIGHT BEHAVES LIKE WAVE - DIFRACTION EXPERIMENT and PARTICLE – PHOTOELECTRIC EFFECT de Broglie WHAT WE CONSIDER PARTICLES CAN BEHAVE LIKE BOTH ALSO. CAN A PARTICLE (ELECTRON) BEHAVE LIKE WAVE? 1 de Broglie YES WITH WAVELENGTH GIVEN BY h λ= P P = momentum= mv 2 DAVISSON GERMER EXPERIMENT ELECTRONS WITH KNOW ENERGY ARE FIRED AT CRYSTAL TARGET. 3 MEASURE NUMBER OF ELECTRONS AT DETECTOR AS FUNCTION OF ANGLE FOR DIFFERENT ENERGIES. 4 AT 500 LARGE NUMBER ARRIVE AT DETECTOR. WHY? WAVES CAN INTERFER CONSTRUCTIVELY! BUT PARTICLES? AT 54 eV MORE ELECTRONS. 5 IF de BROGLIE CORRECT WHAT WILL BE WAVELENGTH FOR 54eV ELECTRONS? 1 2 −19 J ) KE = mv = (54eV )(1.6 x10 2 eV SOLVE FOR v 2(54eV )(1.6 x10 v= −19 J ) eV m 6 m v = 4.36 x10 s 6 m p = mv = (9.1x10 kg)(4.36x10 ) s −31 p = 3.97 x10 − 24 6 m kg s 7 deBroglie −34 6.63x10 Js h λ= = P 3.97 x10−24 kg m s λ = 1.66 x10 −10 m 8 FOR “WAVES” REFLECTED OFF LATTICE nλ = d sin Θ WHERE d SPACING BETWEEN LATTICE SITES and n IS THE NUMBER OF THE PEAK 9 nλ = d sin Θ USE n =1 λ = 1.66 x10 d = 2.15 x10 −10 −10 m m WHICH IS THE SPACING FOR NICKEL 10 (1)(1.66 x10−10 m) = (2.15x10−10 m) sin Θ (1.66 x10 −10 m) sin Θ = = 0.77 −10 (2.15 x10 m) THUS Θ = 50 0 11