Problem 15.1 a. To Find: Elastic modulus and tensile strength of poly
advertisement

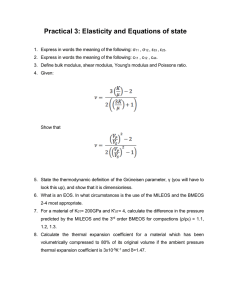
HW 10 Problem 15.1 a. To Find: Elastic modulus and tensile strength of poly(methyl methacrylate) at room temperature [20°C (68°F)]. Compare these with the corresponding values in Table 15.1. b. Given: Figure 15.3 is accurate; the elastic modulus and tensile strength have to be evaluated at room temperature [20°C (68°F)] c. Assumptions: Fig.15.3 is accurate; the material poly(methyl methacrylate) is pure. d. Solution: The elastic modulus is the slope of the linear elastic region of the 20C curve in Fig. 15.3. E = (stress) 30 MPa 0 MPa = = 3.3 GPa (483,000 psi) (strain) 9 103 0 The value for E as per Table 15.1 lies in the range 2.24 to 3.24 GPa (325,000 to 470,000 psi). Thus, the plotted value of E is slightly higher but still close enough to the given value(s). The tensile strength corresponds to the maximum value of stress on the engineering stress vs. engineering strain curve. Here, this is the stress at which the curve ends, i.e., 52 MPa (7500 psi). The range given in Table 15.1 is 48.3 to 72.4 MPa (7000 to 10,500 psi). The value estimated from the plot falls within this range. E = 3.3 GPa Tensile Strength = 52 MPa Problem 15.6 a. To Find: Er(10) for the given viscoelastic polymer b. Given: t 1. Stress decays with time according to the following relation: (t) = (0) exp 2. 0 = 0.6 3. (0) = 2.76 MPa (400 psi) 4. At t = 60s , (t) = 1.72 MPa (250 psi) [ i.e., (60) = 1.72 MPa ] c. Assumptions: 1. The stress decays according to the given relation 2. The values of (0) and (t) and 0 are measured accurately 3. The strain is maintained at a constant value after the polymer is suddenly pulled in tension d. Solution: t Step 1: To determine the value of in the expression (t) = (0) exp 1.72 = 2.76 exp (-60/) exp (-60/) = 1.72/2.76 Taking natural log on both sides: (-60/) = ln(1.72/2.76) => = 126.88 s Step 2: Er(t) = (t) / 0 (Equation 15.1) At t = 10s, equation 15.1 becomes: Er(10) = (10) / 0 The value of (t) at t=10s, i.e., the value of (10) can be evaluated using the given stress decay t equation: (t) = (0) exp , (t) (10) = 2.76 exp (-10/126.88) = 2.55 MPa Er(10) = 2.55/0.6 = 4.25 MPa 4.25 MPa Problem 15.10 a. To Find: (a) schematic plot of the logarithm of relaxation modulus versus temperature as a function of molecular weight (b) schematic plots of the logarithm of relaxation modulus versus temperature as a function of cross-linking b. Given: 1. The polymer is amorphous 2. The variation of the logarithm of relaxation modulus with temperature for an amorphous polymer is schematically represented by Curve C in Fig. 15.8. c. Assumptions: 1. The polymer is indeed amorphous 2. Curve C (in Fig. 15.8) accurately represents the variation of the logarithm of relaxation modulus with temperature for an amorphous polymer d. Solution: (a) Fig.1. Log relaxation modulus as a function of molecular weight and temperature Increasing molecular weight increases the glass-transition and melting temperatures. (b) An increase in the degree of crosslinking leads to an increase in the modulus in both glassy and rubbery regions. Fig1. Effect of increased cross-linking on log relaxation modulus The increase in the degree of cross-linking may also increase the glass transition temperature and, at a high density of cross-links, substantially reduce softening associated with glass transition and prevent melting (rubbery region extends up to the temperature at which the polymer decomposes). Fig1. Effect of increased cross-linking on log relaxation modulus Both Fig.1 and Fig.2. are valid answers Problem 15.19 a. To Find: (1) whether or not it is possible to decide which has the higher tensile modulus (E); (2) if it is possible, which has the higher tensile modulus and why (3) if it is not possible, why not for the given pairs of polymers b. Given: Descriptions of three pairs of polymers c. Assumptions: (1 ) The data regarding the polymers is accurate (2) The polymers are pure d. Solution: No (a) Not possible. The random acrylonitrile-butadiene copolymer will be likely to have to have a lower degree of crystallinity compared to the alternating acrylonitrile-butadiene copolymer since random copolymers do not usually crystallize. The higher the degree of crystallinity, the higher is the tensile modulus of the polymer. Hence, on this basis, it appears that the alternating material would have a higher modulus. However, the random copolymer has a higher degree of crosslinking (10% versus 5% for the alternating copolymer). An increase in crosslinking leads to an increase in the tensile modulus. On this basis, it appears that the random copolymer would have the higher tensile modulus. Thus, it is not possible to determine which would have the higher tensile modulus. (b) Yes It is possible. Linear polymers are more likely to crystallize that branched polymers. The higher the degree of crystallinity, the higher is the tensile modulus of the polymer. The possibility of crystallization for both syndiotactic and isotactic polypropylene is similar, and, thus, the degree is crystallization is not a factor in determining the material with higher tensile modulus. Also, the tensile modulus is nearly insensitive to the degree of polymerization (i.e., molecular weight). Hence, the fact that branched polypropylene has a higher molecular weight does not come into consideration. Hence, the linear and isotactic polypropylene will have a greater tensile modulus. No (c) Not possible. Linear polymers have a higher degrees of crystallinity compared to branched polymers. The higher the degree of crystallinity, the higher is the tensile modulus of the polymer. On this basis, it appears that poly(vinyl chloride) should have a higher value of the tensile modulus. Each repeat unit of poly(vinyl chloride) has 3 of its 4 bonding sites occupied by H atoms whereas 1 site is occupied by Cl. In polyethylene, 4 bonding sites are occupied by H atoms. The atomic radius of Cl is greater than that of H and hence, poly(vinyl chloride) has a more complex repeat unit structure. The bulkier the repeat unit, the lower is the crystallinity. Hence, on this basis, it appears that polyethylene would have a higher degree of crystallinity and hence, a greater tensile modulus. Also, tensile modulus is relatively independent of number-average molecular weight. Therefore, it is not possible to determine which of the two materials has the greater degree of crystallinity, and hence the higher tensile modulus. Problem 15.34 a. To Find: (1) whether or not it is possible to decide which has the higher melting temperature (Tm); (2) if it is possible, which has the higher melting temperature and why (3) if it is not possible, why not for the given pairs of polymers b. Given: Descriptions of three pairs of polymers c. Assumptions: (1 ) The data regarding the polymers is accurate (2) The polymers are pure d. Solution: (a) Yes Yes, it is possible. The lower the branching, the higher is the density and the higher is the melting temperature. Also, the higher the molecular weight, the higher is the melting temperature.Isotactic polystyrene has a higher density. It also has a higher molecular weight. Hence, it will have the higher melting temperature. Yes (b) Yes, it is possible. The presence of larger side group (methyl) in polypropylene makes molecules less flexible , thereby increasing the Tm. Also, larger degree of polymerization (i.e., longer the chain), higher is the Tm. Hence, polypropylene will have the higher Tm . . (c) No No, it is not possible. The repeat unit of polystyrene has a bulkier side group compared to polypropylene. On this basis it appears that polystyrene should have the greater Tm. However, polystyrene has more branching and hence, lower density and a lower degree of polymerization. Both these factors lead to a decrease in its melting temperature . Hence, it is difficult to predict which of the two would have a higher value of Tm.