X Ray Flourescence(XRF) XRF Technique
advertisement

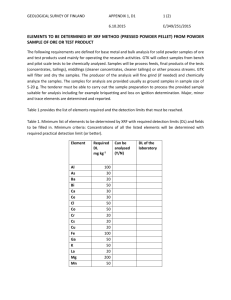
10/27/2009 X‐Ray Flourescence (XRF) XRF Technique •XRF is a rapid, relatively non‐destructive process that produces chemical analysis of rocks, minerals, sediments, fluids, and soils •It’s purpose is to identify the elemental abundances of the sample ‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Aspiring Geologist Phillips PW1606 X‐ray Fluorescence Spectrometer •Indentifies both major and t trace elements l t •It can quantify a broad range of amounts. •From 100% X •Ex) Gold Impurities •To a few parts per million X •Ex) Basalt Flows http://en.wikipedia.org/wiki/File:LDAutoXRFPic.jpg How It Works •Similar process to X‐Ray Spectroscopy and XRD Schematic Representation of XRF X‐Ray •The sample is zapped by short wavelength X‐Rays or Gamma Rays •This radiation excites the sample and dislodges inner orbital electrons causing ionization of the sample •With space in the lower orbitals open, electrons in higher orbitals fall into the lower ones Fluorescent Radiation •This releases a secondary radiation, the fluorescense, from the sample Modified from http://en.wikipedia.org/wiki/X‐ ray_fluorescence How It Works (cont) •Fluorescent radiation is energy released as photons from the sample as electrons move down to lower orbitals to stabilize the atoms. Characteristic Radiation Primary Radiation Secondary Radiation •Energy is released during this process because the binding energy of a low orbital is less than that of a higher orbital. •The energy released is roughly equal to the difference in the binding energies of the two orbitals involved •Both the energy and the wavelengths of the secondary radiation is much less than the original X‐Ray http://www.amptek.com/xrf.html 1 10/27/2009 How It Works (cont) Typical XRF Graph With Spikes •Characteristic of the secondary radiation such as energy and wavelength are specific to element whose atom they were released from •These can be detected and converted into computer generated data •The XRF machines output, coupled with a little quantitative analysis, will report what percent of each element is within the sample http://en.wikipedia.org/wiki/X‐ray_fluorescence Specifics of XRF Wavelength Dispersive Spectrometer •Because the secondary radiation produces such weak photons, the tubes that involve radiation transport must be kept in a vacuum •After secondary radiation occurs, the fluorescence is channeled into a solid state detector, like a crystal, that is able to produce a steady beam of photons for further detection •Specific wavelengths will come off the crystal at specific angles, which allows for isolated detection •This technique is called Wavelength Dispersive Spectroscopy (WSD) Isolating Individual Elements •WSD machines have many detectors, and the capability of isolating individual wavelengths of fluorescent radiation fluorescent radiation http://en.wikipedia.org/wiki/X‐ ray_fluorescence http://en.wikipedia.org/wiki/X‐ray_fluorescence Detection Principles •Complicated process that varies with each of the many detection methods •Detectors contain their own, specific atoms •The secondary radiation will ionize these atoms contained in the detectors producing a charge th d t t d i h •The charge is proportional to the incoming photons energy •Next the charge is collected and the process starts over for the next photon 2 10/27/2009 Detectors Where The K And L Spectra Come From •Two very common types of detectors used to measure the intensity of the secondary radiation are Gas Flow Proportional and Scintillation detectors •Gas Flow Proportional detectors are used to measure smaller wavelengths (K spectra of elements less than Zn(30)). •They are filled with gas(90% Argon), and when the photons pass through, the argon is ionized and the electric field multiplies the charge into a measurable pulse measurable pulse •Scintillation detectors analyze shorter wavelengths (K spectra from NB(41) – I(53) and L spectra from Th(90) – U(92)) •An embedded crystal produces scintillations that are proportional to the energy of the photon absorbed. This pulse is translated into a voltage proportional to the original photon http://en.wikipedia.org/wiki/File:CharacteristicRadiati on.svg Last Step Typical XRF Detection Arrangement •Ideally, experimenters can compare their results to a standard whose composition is very similar •The standards elemental abundance has been calculated by other techniques and is considered accurate http://omega.physics.uoi.gr/xrf/english/the_xrf_technique.htm Sample Preparation •Because preparation is relatively cheap and easy, XRF is a widely used technique •You at least 1 gram of uncontaminated sample Powdered Disk Preparation •The sample must be grinded into a completely homogeneous powder •The Powder is then fused and pressed into a pellet that will adequately absorb the primary X‐Rays •This technique is best suited for trace element analysis • One type of preparation technique involves converting the powdered sample into disks powdered sample into disks •The other common preparation technique consists of converting the sample into a glass bead •Some labs do both, others use only one technique http://www.nbmg.unr.edu/lab/xrf.htm 3 10/27/2009 Glass Bead Preparation Preparation (cont) •The sample is melted in a 1000 Ԩ furnace •The flux in the glass bead makes it very durable •It is added to a flux. For example Washington State uses a 2:1 ratio of di‐lithium tetraborate flux to sample •The glass bead samples can survive many decades of analysis •Lithium and Boron are commonly used in fluxes because they are light and absorb few X‐Rays •After cooling, the glass is shaped and polished into a bead suitable for XRF analysis Calibration •Two approaches: empirical and fundamental parameters •Empirical calibration is based on the analysis of known standards. Standards should have similar elemental abundances to the sample •Fundamental Fundamental Parameters Calibration may be done for standardless Parameters Calibration may be done for standardless samples •It relies on built‐in algorithms that refer to the physics of the detector’s response to a sample of one pure element •It may be checked by running a standard after calibration is completed Potential Error •Any standards used in comparison with XRF results must be prepared the same way the sample was •There is usually a correction made after the initial XRF analysis and using a glass sample can make this process easier •By using a “watered down” sample, the correction is much smaller Limitations •Cannot analyze elements lighter than Na(11) •Cannot distinguish between various isotopes of elements •Cannot distinguish between different ions of the same element •Most machines have some limitations on elemental analysis and cannot identify every element with atomic mass > 11 Practical Uses •Calibration mistakes can throw of the results •Improper sample preparation •Mechanical failure of the machine •Improper orientation of critical parts •Crystals • detectors • X‐ray beams •XRF is used in the commercial production of metals, glasses, ceramics, and building materials •Utilized in research fields such as geochemistry!, archaeology, construction, forensic science, environmental studies, the , , , petroleum industry, and mining 4 10/27/2009 Ray Gun? No it’s a handheld XRF! Sources Papachristodoulou, Christina. “Elemental analysis using the XRF Technique.” XRF Laboratory. 27 Sept 2009 <http://omega.physics.uoi.gr/xrf/english/the_xrf_technique.htm > Rollinson, Hugh. Using Geochemical Data. Essex: Pearson Limited, 1993. Wirth, Karl, and Andy Barth. “X‐Ray Fluorescence.” Geochemical Instrumentation and Analysis. Carleton College. 24 Sept. 2009 <http://serc.carleton.edu/research education/geochemsheets/te chnique <http://serc.carleton.edu/research_education/geochemsheets/te s/XRF.html> “X‐ray Fluorescence.” Wikipedia. 2 Oct 2009 ray_fluorescence> <http://en.wikipedia.org/wiki/X‐ “X‐Ray Fluorescence Spectroscopy (XRF).” Amptek. 4 Oct 2009 <http://www.amptek.com/xrf.html> http://www.rcon‐ndt.com/xrf.html Gneiss XRF Machine! Panalytical Epsilon 5 http://www.noc.soton.ac.uk/geochem/Facilities%20Links/xrf.ht m 5