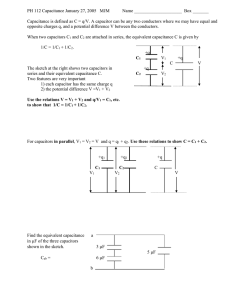
calculation method of specific capacitance
advertisement

Supplementary Material (ESI) for Chemical Communications This journal is (c) The Royal Society of Chemistry 2010 Electronic Supplementary Information Enhanced capacitance of manganese oxide via confinement inside carbon nanotubes Wei Chen,a Zhongli Fan,a Lin Gu,b Xinhe Bao,c and Chunlei Wang*a a Department of Mechanical and Materials Engineering, Florida International University, Miami, FL 33174, USA b Stuttgart Center for Electron Microscopy, Max-Planck Institute for Metals Research, Stuttgart 70569, Germany, and c State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, The Chinese Academy of Sciences, Dalian 116023, P. R. China. * Corresponding author. E-mail: wangc@fiu.edu S1 Supplementary Material (ESI) for Chemical Communications This journal is (c) The Royal Society of Chemistry 2010 SI1. Preparation and Characterization of MnO2-in-CNT and MnO2-out-CNT Raw CNTs (Length 10−50 μm, inner diameter 4−8 nm and outer diameter 10−20 nm, purchased from Cheap Tubes Inc.) were first opened up and cut into segments of 0.2−1 μm in length by refluxing in HNO3 (68 wt. %) at 140 ºC for 14 h. The metal catalyst residues were removed during this process. The resulting CNTs with open tips and functionalized carbonyl groups are denoted as oCNTs. The oCNTs were immersed into an aqueous Mn(NO3)2 (50% w/w aq., Alfa Aesar) solution, which was introduced into the CNT channels utilizing the capillary forces of CNTs aided by ultrasonic treatment and stirring. After the resulting slurry mixture was dried at room temperature, it was gradually heated to 210 ºC and held for 1 h. By this process, Mn(NO3)2 decomposes into β-MnO2 and the obtained sample is denoted as MnO2-in-CNT. The loading of MnO2 is investigated from 10, 12, 15 to 18 wt. %. In order to fill the nanochannels of CNTs utilizing the capillary forces efficiently, we have confirmed that the loading less than 20 wt. % can be reproducibly controlled. For comparison, the same loadings of MnO2 were deposited on the outer surface of nanotubes by impregnating cCNTs with aqueous Mn(NO3)2 solution. This cCNTs with closed caps were obtained by refluxing raw CNTs in 37 wt. % HNO3 solution at 110 ºC for 5 h. Then, MnO2-out-CNT was obtained after the same drying procedure. As shown in the Figure S1, the specific capacitance of MnO2-out-CNT increase monotonously with the MnO2 loading increasing from 10 to 18 wt.% (Figure S1 A). The corresponding MnO2 normalized capacitance reach the highest value at 15 wt.% (Figure S1 B). For the sample MnO2-in-CNT, the specific capacitance of composite exhibits the highest value (225 F/g) while the MnO2 normalized capacitance is the highest at 12 wt.%, and then slightly decrease when the loading increase to 15 wt.%. Based on the comprehensive results, we choose MnO2 loading of 15 wt.% for both samples MnO2-in-CNT and MnO2-out-CNT unless otherwise stated. Fig. S1 Influence of MnO2 loading on the specific capacitance of (A) the composites MnO2-out-CNT and MnO2-in-CNT and (B) MnO2 normalized. S2 Supplementary Material (ESI) for Chemical Communications This journal is (c) The Royal Society of Chemistry 2010 The reference MnO2 was prepared by the similar process without the additive of CNTs. Transmission electron microscopy was performed using a Philips CM200 FEG microscope operating at 200 kV. HAADF analysis was performed using the Zeiss SESAM (Carl Zeiss, Oberkochen, Germany) microscope equipped with an electrostatic Ω-type monochromator and the MANDOLINE filter. TEM pictures of MnO2-in-CNT and MnO2-out-CNT are shown in Figure S2. Fig. S2 TEM micrographs of the samples (a) MnO2-in-CNT and (b) MnO2-out-CNT. SI2. Raman characterization Raman spectroscopy measurements were carried out with an argon ion (Ar+) laser system (Spectra Physics, model 177G02) of λ = 514.5 nm at a laser power of ca.7 mW. The samples Mn2O3-out-CNT, Mn2O3-in-CNT and Mn2O3 were obtained by reduction of MnO2-out-CNT, MnO2-in-CNT and the reference MnO2 in flowing 5% H2/N2 up to 250 ºC for 1 h at a rate of 2 ºC min-1. S3 Supplementary Material (ESI) for Chemical Communications This journal is (c) The Royal Society of Chemistry 2010 Fig. S3 Raman Spectra of (a) Mn2O3-out-CNT, (b) Mn2O3-in-CNT, and (c) Mn2O3. The ratio of disorder band and graphite band (G mode, around 1590 cm-1) is generally used to describe the amorphous carbon and graphitized structure in the samples. It can be obtained from Fig. 2A a, b, c & d that ID/G values for cCNTs, oCNTs, MnO2-out-CNTand MnO2-in-CNT are 1.1, 0.9, 1.1 and 1.0, respectively. This means that there are more disorder carbon (structural defects) in the samples cCNTs and MnO2-out-CNT. In general, disorder carbon can contribute relatively larger surface area compared to graphite structure, which should be beneficial for the capacitive performance.1,2 However, the specific capacitance of cCNTs is lower than that of oCNTs though ID/G of cCNTs (1.1) is higher than that of oCNTs (0.9). This indicates the contribution of structural defects is negligible. SI3. Calculation method of specific capacitance from CV To quantitatively evaluate the charge storage capacity, the specific capacitance of the composites is determined by the expression Voltammetric charge/(potential window × composite loading).3,4 Considering that the anodic voltammetric charges (qa) and cathodic voltammetric charges (qc) are not same in that the shape of CV curves is not the idea mirror-symmetry, we use integral area of CV curve/ scan rate represent the sum of anodic and cathodic voltammetric charges, i.e., Voltammetric charge.5-7 S4 Supplementary Material (ESI) for Chemical Communications This journal is (c) The Royal Society of Chemistry 2010 So the specific capacitance is calculated accurately on the basis of the following equation: C = ∫ E2 E1 i ( E ) d E / 2 ( E 2 − E 1 ) mν where C is the specific capacitance of individual sample. E1, E2 are the cutoff potentials in cyclic voltammetry. i(E) is the instantaneous current. ∫ E2 E1 i(E)dE is the total voltammetric charge obtained by integration of positive and negative sweep in cyclic voltammograms. (E2 – E1) is the potential window width. m is the mass of individual sample, which is the mass difference of the working electrode before and after electrospray deposition (vide post). We used a METTLER TOLEDO® balance with an accuracy of 0.01 mg to weigh the mass of the sample. ν is the potential scan rate. There are two different kinds of capacitive contributions: one is the double-layer capacitance from CNTs, the other is the pseudocapacitance from MnO2. The former stores charge electrostatically and is determined by surface area. While, for the latter, charge is stored in virtue of highly reversible redox reactions between Mn(IV)/Mn(III) species. Supposing the surface area of CNTs in the composites MnO2/CNTs has not change significantly, the double-layer capacitance of the composite is close to that of blank CNTs. Then, we get the pseudocapacitance of MnO2 by subtracting the CNT double-layer capacitance from the total capacitance.The specific capacitance based on MnO2 (i.e. MnO2 normalized capacitance) is obtained from the CV curves by deducting the capacitance of CNTs from total capacitance of manganese oxide/CNTs composites following the expression (total capacitance of the composites – capacitance of CNTs)/ MnO2 loading.8,9 Further work will be done to consider more detailed circuit model. SI4. ESD Experimental section The samples were deposited on glassy-type pyrolytic carbon substrate by means of Electrostatic Spray Deposition (ESD). Pyrolytic potoresist carbon film with thickness of ~1.5 μm was prepared on a Si substrate. In brief, a silicon wafer was spin coated with photoresist AZ4620 (MicroChem), then pyrolyzed at 1000 ºC for 1 h in Ar atmosphere. The ESD setup was shown in Figure S3. A high dc voltage of 8 kV was applied between glassy carbon substrate and syringe containing the sample solution, which was atomized so as to generate a spray at the tip of the needle. The feeding rate was 5 mL h-1. The atomized droplets were sprayed to the heated substrate (140 ºC) by the electrostatic force, thereby forming an uniform thin layer of samples on the substrate surface. An additional ultrasonic oscillation was conducted during spray process to ensure the uniformity of the solution. S5 Supplementary Material (ESI) for Chemical Communications This journal is (c) The Royal Society of Chemistry 2010 The thin film electrodes prepared by the ESD enable us to evaluate the electrochemical properties of the active materials themselves, because the electrodes do not include the binders and conductive additives which are usually used for the fabrication of composite electrodes. Fig. S4 Schematic of ESD experimental setup. SI5. The electrochemical performance of reduced samples Cyclic voltammetry (CV) was performed using a CHI 660C workstation (CH Instrument, USA) with a typical three-electrode cell equipped with a N2 gas flow system. The above samples deposited on pyrolytic photoresist carbon substrates were used as working electrodes, an Ag/AgCl (3 M NaCl-filled) electrode as a reference electrode, and a platinum wire as a counter electrode. The electrolyte was 1 M Na2SO4 aqueous solution. The specific capacitance of Mn2O3-in-CNT and Mn2O3-out-CNT is 74 and 73 F g-1 at 2 mV s-1, respectively. S6 Supplementary Material (ESI) for Chemical Communications This journal is (c) The Royal Society of Chemistry 2010 Fig. S5 Cyclic Voltammetry curves of Mn2O3-out-CNT (dotted line) and Mn2O3-in-CNT (dash dotted line) at scan rate 2 mV s-1 in 1 M Na2SO4. References 1 A. Janes, H. Kurig, E. Lust, Carbon 2007, 45, 1226. 2 N. M. Pontikos, R. L. McCreery, J. Electroanal. Chem. 1992, 324, 229. 3 C. C. Hu, T. W. Tsou, Electrochem. Commun. 2002, 4, 105. 4 B. E. Conway, Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications, Kluwer, New York, 1999, p21. 5 H. Li, J. Wang, Q. Chu, Z. Wang, F. Zhang, S. Wang, J. Power Sources 2009, 190, 578. 6 C. C. Wang, C. C. Hu, Carbon, 2005, 43, 1926. 7 K. W. Nam, C. W. Lee, X. Q. Yang, B. W. Cho, W. S. Yoon, K. B. Kim, J. Power Sources 2009, 188, 323. 8 Z. Fan, J. Chen, M. Wang, K. Cui, H. Zhou, Y. Kuang, Diam. Relat. Mater. 2006, 15, 1478. 9 S. L. Chou, J. Z. Wang, S. Y. Chew, H. K. Liu, S. X. Dou, Electrochem. Commun. 2008, 10, 1724. S7