X-ray Powder Diffraction II Peak Intensities
advertisement

X-ray Powder Diffraction II
Peak Intensities
Chemistry 754
Solid State Chemistry
Lecture #10
Outline
• Elastic scattering of X-rays by atoms
• Diffraction peak intensities
–
–
–
–
Structure Factors
Multiplicity
Lorentz and Polarization Factors
Temperature Factors
• Neutron diffraction
• Electron diffraction
1
Interaction of X-rays with Matter
These are the
diffracted X-rays
Elastic Scattering by an Electron
• Charged particles (electron) scatter
electromagnetic radiation (x-rays)
– The varying electric field of the X-ray induces an oscillation
of the electron
– The oscillating electron then acts as a source of
electromagnetic radiation
– In this way the x-rays are scattered in all directions
• JJ Thompson analyzed the scattering and found
that:
I = I0[(μ0/4p)2(e4/m2r2)sin2α]
I = I0(K/r2) sin2α
α is the angle between the scattering direction and the
direction in which the electron is accelerated
r is the distance from the scattering electron
K is a constant
2
Scattering by an Atom
We can consider an atom to be a collection of
electrons. The electrons around an atom scatter
radiation in the manner described by Thompson.
However, due to the coherence of the radiation we
need to consider interference effects from different
electrons within an atom. This leads to a strong
anglular dependence of the scattering. We express
the scattering power of an atom by its form factor (f).
X-ray Form Factors
25
The form factor is
equivalent to the atomic
number at θ = 0
Form Factor, f
20
Ca
Ca2+
15
10
The form factor
drops rapidly as a
function of (sin θ)/λ
5
0
0
0.1
0.2
0.3
0.4
0.5
0.6
Sin(θ)/λ
3
The Effect of Form Factors on
Diffraction Patterns
4500
TbBaFe2O5 - 70 K
Neutron Data
TbBaFe2O5 - 300 K
Synchrotron X-ray
4000
Intensity (Arb. Units)
3500
Intensity
3000
2500
2000
1500
1000
500
0
5
25
45
65
2-Theta (Degrees)
The peak intensities drop off at
high angles in an X-ray diffraction
pattern because the form factor
decreases
10
30
50
70
90
110
130
2-Theta (Degrees)
Neutrons are scattered from the
nucleus and the form factor is not
angle dependent. Intensities do
not drop off at high angle.
Diffraction Intensities
The integrated intensity (peak area) of each powder
diffraction peak is given by the following expression:
I(hkl) = |S(hkl)|2 × Mhkl × LP(θ) × TF(θ)
–
–
–
–
S(hkl) = Structure Factor
Mhkl = Multiplicity
LP(θ) = Lorentz & Polarization Factors
TF(θ) = Temperature factor (more correctly
referred to as the displacement parameter)
This does not include effects that can sometimes by
problematic such as absorption, preferred orientation
and extinction.
4
Structure Factor
The structure factor reflects the interference between atoms in
the basis (within the unit cell). All of the information regarding
where the atoms are located in the unit cell is contained in the
structure factor. The structure factor is given by the following
summation over all atoms (from 1 to j) in the unit cell:
S(hkl) = Σj fj exp {-i2π(hxj+kyj+lzj)}
– fj = form factor for the jth atom
– h, k & l = Miller indices of the hkl reflection
– xj, yj & zj = The fractional coordinates of the jth atom
To evaluate the value of the imaginary term in the exponential
function, remember Euler’s equation:
exp (-ix) = cos(x) – i sin(x)
The value of this function is a real number when x is a multiple of
2π. It is equal to 1 for even multiples of 2π and -1 for odd
multiples of 2π.
Structure Factors: Example CsCl
• Let’s calculate the structure factors for the first 6
peaks CsCl. To do this we need to know the atomic
positions and the Miller Indices.
Cl at (0,0,0)
Cs at (½,½,½)
5
Structure Factors: Example CsCl
S(hkl) = Σj fj exp {-i2π(hxj+kyj+lzj)}
S(hkl) = fCl exp {-i2π([h×0]+[k×0]+[l×0]}
+ fCs exp {-i2π([h×½]+[k×½]+[l×½]}
S(hkl) = fCl exp {0} + fCs exp {-iπ(h+k+l)}
S(hkl) = fCl – fCs when h+k+l is an odd number
S(hkl) = fCl + fCs when h+k+l is an even number
S(100) = fCl - fCs
S(111) = fCl - fCs
S(210) = fCl - fCs
S(110) = fCl + fCs
S(200) = fCl + fCs
S(211) = fCl + fCs
Systematic Absences: Body Centering
Recall that earlier we said that for a body centered space
group all of the peaks where h+k+l is an odd number will
be missing. Consider the structure factors for sodium
which has the BCC structure with Na atoms at (0,0,0) and
(½,½,½). The equations will be just like CsCl, but the two
form factors will be equal.
S(hkl) = fNa exp {-i2π([h×0]+[k×0]+[l×0]}
+ fNa exp {-i2π([h×½]+[k×½]+[l×½]}
S(hkl) = fNa exp {0} + fNa exp {-iπ(h+k+l)}
S(hkl) = fNa – fNa = 0 when h+k+l is an odd number
S(hkl) = fNa + fNa = 2fNa when h+k+l is an even number
6
Int.
Na (BCC)
(110)
12000
10000
4000
(220)
(200)
6000
(211)
8000
2000
Simulated X-ray
powder
diffraction
patterns
[Calculated with
Diamond]
0
20
30
40
2Theta
50
60
Int.
(210)
(220)
(110)
20000
(200)
30000
(111)
40000
(100)
50000
(211)
CsCl
60000
10000
0
20
30
40
2Theta
50
60
Multiplicity Factor
In a powder diffraction experiment the d-spacings for related
reflections are often equivalent. Consider the examples below:
Cubic
– (100), (010), (001), (-100), (0-10), (00-1) → Equivalent
Multiplicity Factor = 6
– (110), (-110), (1-10), (-1-10), (101), (-101), (10-1),
(-10-1), (011), (0-11), (01-1), (0-1-1) → Equivalent
Multiplicity Factor = 12
In general for a cubic system where the Miller indices are n1, n2 and
n3 (all unequal) the multiplicity factors Mhkl are:
n100 (i.e. 100) → M = 6
n1n10 (i.e. 110) → M = 12
n1n1n2 (i.e. 221) → M = 24
n1n1n1 (ie 111) → M = 8
n1n20 (ie 210) → M = 24
n1n2n3 (ie 321) → M = 48
The multiplicities are lower in lower symmetry systems. For example
in a tetragonal crystal the (100) is equivalent with the (010), (-100)
and (0-10), but not with the (001) and the (00-1).
7
Lorentz Factor
There are a number of factors that lead to a theta
dependence of the peak intensities (integrated
intensities).
• Diffraction can occur for angles slightly different from the value
predicted by Bragg’s Law → I α 1/sin(2θ)
• The number of crystals oriented in such a way as to satisfy Bragg’s
Law is highest for low angles → I α cos(θ)
• The fraction of the diffraction cone that intersects the detector is
highest at low angles → I α 1/sin(2θ)
When combine these considerations and do some
trigonometric manipulation we get the Lorentz Factor:
I α 1/(4sin2θ cosθ)
Polarization and the LP Factor
An X-ray propagating in the x-direction will have an
electric vector oriented in the yz plane. The y and z
components of the X-ray will be scattered differently
because the angle between the scattered beam and the
electric field gradient will differ, as derived by
Thompson. This leads to the polarization factor:
I α (1+cos22θ)/2
Typically the Lorentz and Polarization terms are combined
to give the Lorentz-Polarization (LP) factor.
I α (1+cos22θ)/(8sin2θ cosθ)
8
Temperature Factor
The vibrations of atoms in a crystal lead to an angle dependent effect
on the diffracted peak intensities. The more an atom vibrates the
scattering power is decreased, because the scattering power of the
atom is smeared out. As an approximation we can assume that all atoms
vibrate equally. In that case the TF can be expressed by the equation:
TF(θ) = exp {B(sin θ/λ)2}
Where the coefficient B is the isotropic temperature factor.
– B is proportional to the mean squared displacement of the atoms
– Typical values for inorganic extended solids range from 0.5 to 1.5.
– The temperature factor has its biggest impact at high angles.
More sophisticated treatments assume
– Different values of B for each crystallographically independent atom
– Anisotropic vibrations (elliptical in shape rather than spherical).
5
1.2
Lorentz-Polarization
4
3.5
1
0.8
3
2.5
0.6
2
0.4
1.5
1
Temperature Factor
LP
LP Inc. Beam Mono
Temp. Factor (B=1)
Temp. Factor (B=2)
4.5
0.2
0.5
0
0
50
100
2-Theta
150
0
200
9
Int.
(220)
(210)
20000
(200)
30000
(111)
40000
(211)
CsCl
(110)
50000
(100)
Intensity
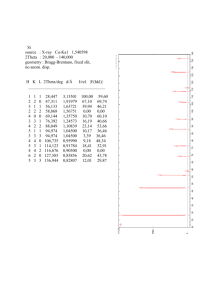
Calculations
for CsCl
60000
10000
0
20
h
k
l
0
0
1
0
0
1
0
0
1
1
0
1
1
2
1
1
1
2
2
2
2
2Theta [°] d-spacing [Å]
21.54
30.64
37.76
43.88
49.39
54.47
63.80
4.123
2.915
2.380
2.062
1.844
1.683
1.458
30
40
2Theta
S
LP
34.66
58.72
31.54
51.98
29.32
47.68
44.64
6.80
3.23
2.05
1.47
1.12
0.90
0.63
50
60
Mult. Relative Int.
6
12
8
6
24
24
12
37
100
12
18
17
37
11
Neutron Diffraction
Diffraction from crystals can also be carried out with neutron and
electrons. The basic concept are the same though there are
important distinctions. Let’s begin with the DeBroglie relationship.
λ = h/p = h/mv
but the kinetic energy, E, and velocity, v, of a particle are related by
the expression
E = (1/2)mv2 → v = sqrt(2E/m)
combining the two relationships gives
λ = h/sqrt(2mE)
finally for thermal neutrons from a reactor source the energy of an
electron is E = kT so that
λ = h/sqrt(2kT)
Typical values for a neutron reactor source are 300<T<400K and 1.0
A< λ < 2.5 A.
10
Reactor Diffractometers
Thermal Neutron Instruments at NIST
BT1 Powder Diffractometer
BT1 Powder Diffractometer at NIST
11
X-rays vs. Neutrons
• Neutron beams are highly penetrating (penetration depths of
centimeters-decimeters), whereas X-rays are highly attenuated
by matter (penetration depths of microns-millimeters).
– Larger samples are needed for neutron diffraction
• Neutrons are scattered from nuclei, whereas X-rays are
scattered by electrons
– Neutron scattering factors do not drop off with increasing θ
• Neutron scattering power varies irregularly with atomic number
and mass number, whereas X-ray scattering power varies
smoothly with atomic number.
– Neutrons are more sensitive to light elements (H, N, O, F, C, etc.)
and isotope distributions
– Some elements strongly absorb neutrons and cannot be easily
studied (B, Cd, Sm, Eu, Gd, Dy, Hf, Ir, Hg), some are essentially
transparent to neutrons (V) or have negative scattering lengths (Ti,
Mn)
• Neutrons have an intrinsic magnetic moment, whereas X-rays
have no magnetic moment
– Neutrons are used to determine ordered magnetic structures
Electron Diffraction
Diffraction from crystals can also be carried out with neutron and
electrons. The basic concept are the same though there are
important distinctions. Let’s begin with the DeBroglie relationship.
λ = h/p = h/mv
but the kinetic energy, E, and velocity, v, of a particle are related by
the expression
E = (1/2)mv2 → v = sqrt(2E/m)
combining the two relationships gives
λ = h/sqrt(2mE)
The energy of an electron is related to the accelerating voltage, V,
by the relationship E = eV. Upon correcting for relativistic effects
we get an approximate relationship between the wavelength (in
Angstroms) and the voltage
λ = sqrt(150/V)
Typical values for a electron microscope are V ~ 100 kV and λ ~ 0.04
Angstroms
12
Transmission Electron Microscope
This is the CM300 UltraTwin Field Emission Gun
TEM.
It is located in the Campus
Center for Electron Optics
(CEOF) in the MSE
department
X-rays vs. Electrons
• Electrons are highly attenuated by matter (penetration depth of
nanometers), whereas X-rays are strongly attenuated by matter
(penetration depths of microns-millimeters).
– Data collection done under high vacuum
– Data collection is usually a transmission measurement (TEM) on a thin
sample or crystal
• Electrons beams can be highly focused, whereas X-rays are
difficult to focus
– The electron beam is usually focused onto a very small micro-single
crystal
• Electron scattering power drops off strongly as a function of 2theta, whereas X-ray scattering power drops off smoothly as a
function of 2-theta
– Data sets are often collected over a very limited angular range
(± 4° 2-theta)
• Diffracted electrons can interact strongly with the lattice making
it difficult to get reliable intensities, whereas X-ray intensities and
peak positions can be determined with a great deal of accuracy
– Structure refinement and solution is challenging
13