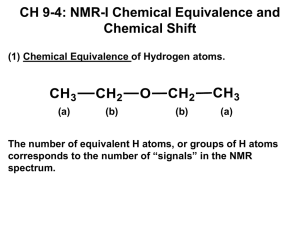
(16 points) Show how many peaks you would expect to observe
advertisement
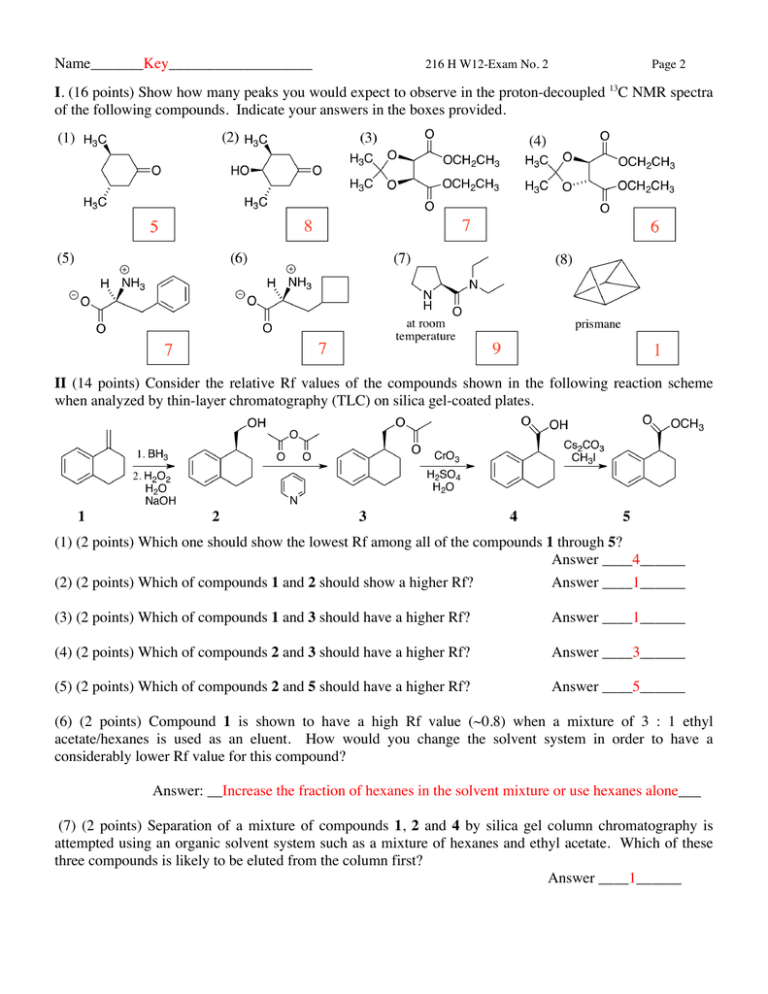
Name_______Key___________________ 216 H W12-Exam No. 2 Page 2 I. (16 points) Show how many peaks you would expect to observe in the proton-decoupled 13C NMR spectra of the following compounds. Indicate your answers in the boxes provided. II (14 points) Consider the relative Rf values of the compounds shown in the following reaction scheme when analyzed by thin-layer chromatography (TLC) on silica gel-coated plates. (1) (2 points) Which one should show the lowest Rf among all of the compounds 1 through 5? Answer ____4______ (2) (2 points) Which of compounds 1 and 2 should show a higher Rf? Answer ____1______ (3) (2 points) Which of compounds 1 and 3 should have a higher Rf? Answer ____1______ (4) (2 points) Which of compounds 2 and 3 should have a higher Rf? Answer ____3______ (5) (2 points) Which of compounds 2 and 5 should have a higher Rf? Answer ____5______ (6) (2 points) Compound 1 is shown to have a high Rf value (~0.8) when a mixture of 3 : 1 ethyl acetate/hexanes is used as an eluent. How would you change the solvent system in order to have a considerably lower Rf value for this compound? Answer: __Increase the fraction of hexanes in the solvent mixture or use hexanes alone___ (7) (2 points) Separation of a mixture of compounds 1, 2 and 4 by silica gel column chromatography is attempted using an organic solvent system such as a mixture of hexanes and ethyl acetate. Which of these three compounds is likely to be eluted from the column first? Answer ____1______ Name_______Key__________________ 216 H W12-Exam No. 2 Page 3 1 III (12 points) Shown below is the akenic H region of the 100 MHz H NMR spectrum of methyl acrylate (5% in C6D6). Determine the chemical shifts of HA, HB, and Hc and the three coupling constants involving these H’s. Write your answers in the box below IV. (12 points) The following are the observed chemical shifts and coupling constants assignable to H-1 ~ H-6 of 7-oxabicyclo[2.2.1]heptane 6. 1 H NMR (360 MHz, CDCl3) δ 2.73 (1H, d, J = 15.0 Hz), 2.86 (1H, ddd, J = 15.0, 6.0, 1.25 Hz), 4.27 (1H, d, J = 4.5 Hz), 4.38 (1H, ddd, J = 5.0, 4.5, 1.25 Hz), 5.00 (1H, dd, J = 6.0, 5.0 Hz), and 5.05 ppm (s, 1H). On the basis of the above NMR information and using the coupling constant information given below, assign all chemical shifts to each of the six hydrogens (H1 ~ H6). Note that there is a W-letter coupling between 3exo-H and 5-H. Name________Key__________________ 216 H W12-Exam No. 2 Page 4 V. (16 points) On the basis of its spectroscopic information provided below, answer the questions given below and propose the structure of the compound, C5H7NO2. -1 IR (KBr): 2913-2850 (several weak peaks), 2270 (sharp, medium intensity), 1601 (s), 1745 (strong) cm (1) (1 point) What is (are) the unit(s) of unsaturation of this compound? _______Three_______ (2) (2 points) What does the IR and 13C NMR information tell you in terms of an O-containing functional group in this molecule? The presence of ____Ester_____ group. (3) (2 points) What does the IR and 13C NMR information tell you in terms of an N-containing functional group in this molecule? The presence of __C≡Ν or Cyano___ group. (4) (11 points) Draw in the box below the structure of the compound (3 points) and assign all 1H and 13C peaks by writing the chemical shift values next to the corresponding 1H and 13C nuclei (8 points). Name________Key__________________ 216 H W12-Exam No. 2 Page 5 VI. (20 points) On the basis of its spectroscopic information provided below, answer the questions given on page 6 and propose the structure of the compound, C10H13NO2. Name__________Key________________ 216 H W12-Exam No. 2 Page 6 VI. (continued) (1) (1 point) What is (are) the unit(s) of unsaturation of this compound? _____Five_________ (2) (2 point) What does the IR information tell you in terms of an O-containing functional group in this molecule? The presence of ____Ester____ group. 1 (3) (2 points) What does the IR and H NMR information tell you in terms of an N-containing functional group in this molecule? The presence of ____NH2_____ group. (4) (2 points) What does a pair of doublets in the 6.5-8 ppm region of the 1H NMR spectrum tell you in terms of the substitution pattern of the benzene ring? __1,4- or para-disubstituted benzene___ (5) (13 points) Draw in the box below the structure of the compound (4 points) and assign all 1H peaks by writing the chemical shift values next to the corresponding nuclei (9 points). 6.6 ppm H 7.9 ppm H O H2N 4.0 ppm 6.6 ppm O H H 4.2 ppm H2 C C H2 CH3 1.0 ppm 7.9 ppm 1.8 ppm 13 VII. (10 points) The chemical shifts of H-1~H-6 and coupling constants involving these 7 Hs of tetraacetate 7 are summarized as follows. δ 2.03 (1H, ddd, J = 13.4, 12.2, 10.5 Hz), 2.33 (1H, ddd, J =12.2, 4.6, 3.3 Hz), 3.04 (1H, ddd, J = 13.4, 4.0, 3.3 Hz), 5.14 (1H, ddd, J = 10.5, 10.5, 4.6 Hz), 5.15 (1H, dd, J = 10.5, 2.5 Hz), 5.38 (1H, dd, J = 4.0, 3.0 Hz), and 5.40 (dd, 1H, J = 3.0, 2.5 Hz) ppm. On the basis of the above NMR information, write the chemical shift of each of H-1 ~ H-5 in the box next to the corresponding H. Note that two C-6 H’s have already been assigned. No W-letter couplings are observed in 7.