Oxidation–Reduction Reactions
advertisement

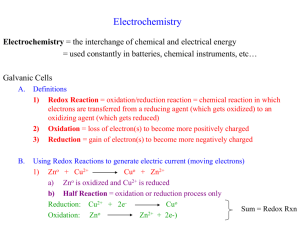
Oxidation–Reduction Reactions Oxidation Loss of electrons Reduction Gain of electrons Zn( s ) + 2 H + ( aq ) → Zn +2 ( aq ) + H 2 ( g ) Zn is oxidized H+ is reduced Zn is the reducing agent H+ is the oxidizing agent reductant oxidant Oxidation Numbers 1. For an atom in its elemental form the oxidation number is zero. 2. For any monoatomic ion the oxidation number equals the charge on the ion. K+, O-2, Al+3, etc 3. Nonmetals usually have negative oxidation numbers. a. the oxidation number of O is usually -2 b. the oxidation number of H is +1 when it is bonded to a non metal (H2O, NH3, H3PO4, etc. ) and -1 when it is bonded to a metal (CaH2, NaH, etc.) c. the oxidation number of F is always -1. The other halogens are usually -1 but when combined with oxygen in oxycations it is +1 ( OCl-1, O2Cl-2, etc. ) “If this were an ionic compound, what would the charges be?” Identify the oxidation numbers H 2O NiO2 − 4 MnO Ni( OH )2 Al( OH )4− −2 4 C2 O NH 3 −2 7 Cr2O ClO3− Balancing Oxidation Reduction Reactions Half-Reaction Method Sn +2 ( aq ) + Fe +3 ( aq ) → Sn +4 ( aq ) + Fe +2 ( aq ) Sn +2 ( aq ) → Sn +4 ( aq ) + 2e − Fe +3 ( aq ) + e − → Fe +2 ( aq ) 2 Fe +3 ( aq ) + 2e − → 2 Fe+2 ( aq ) Sn +2 ( aq ) → Sn +4 ( aq ) + 2e − Sn +2 ( aq ) + 2 Fe+3 ( aq ) → Sn +4 ( aq ) + 2 Fe+2 ( aq ) MnO4− ( aq ) + C2O4−2 ( aq ) → Mn +2 ( aq ) + CO2 ( g ) Acidic solution 2 MnO4− ( aq ) + 5C2O4−2 ( aq ) + 16 H + ( aq ) → 2 Mn +2 ( aq ) + 10CO2 ( g ) + 8 H 2O( l ) 2 MnO4− ( aq ) + 5C2O4−2 ( aq ) + 16 H + ( aq ) → 2 Mn +2 ( aq ) + 10CO2 ( g ) + 8H 2O( l ) MnO4− ( aq ) → Mn +2 ( aq ) + 4 H 2O( l ) 8 H + ( aq ) + MnO4− ( aq ) → Mn +2 ( aq ) + 4 H 2O( l ) 5e − + 8 H + ( aq ) + MnO4− ( aq ) → Mn +2 ( aq ) + 4 H 2O( l ) C2O4−2 ( aq ) → 2CO2 ( g ) C2O4−2 ( aq ) → 2CO2 ( g ) + 2e − 10e − + 16 H + ( aq ) + 2 MnO4− ( aq ) → 2 Mn +2 ( aq ) + 8 H 2O( l ) 5C2O4−2 ( aq ) → 10CO2 ( g ) +10e − 2MnO4− ( aq ) + 5C2O4−2 ( aq ) + 16 H + ( aq ) → 2Mn +2 ( aq ) + 10CO2 ( g ) + 8H 2O( l ) Rules (Acidic Solution) 1. Divide the reaction into half-reactions 2. Balance each half-reaction a. First balance elements other than O & H b. Balance O atoms using H2O c. Balance H atoms with H+ d. Balance charge with e3. Multiply each half reaction by an integer so each has the same number of electrons 4. Add half reactions and simplify 5. Check! Balancing in a basic solution Use OH- & H2O Given CN − ( aq ) + MnO4− ( aq ) → CNO − ( aq ) + MnO2 ( s ) Balanced Equation 3CN − ( aq ) + 2MnO4− ( aq ) + H 2O( l ) → 3CNO − ( aq ) + 2MnO2 ( s ) + 2OH − ( aq ) A Fundamental Observation When a strip of Zn is placed in a solution containing Cu+2(aq) ions the Cu+2(aq) ions react with the Zn and form Cu metal while the Zn dissolves, forming Zn+2(aq) ions. Cu +2 ( aq ) + Zn( s ) → Cu( s ) + Zn +2 ( aq ) Voltaic Cell A device in which a spontaneous oxidationreduction reaction occurs with the passage of electrons through an external circuit. Electrodes Two solid metals connected by the external circuit Anode Electrode at which oxidation occurs Cathode Electrode at which reduction occurs Salt Bridge Electrical contact between the two solutions that prevents them from mixing but permits ion flow Electrodes need not enter into reaction Beaker A H2O, K2Cr2O7, & H2SO4 Beaker B HI & H2O Put Pt electrode in both beakers Connect electrodes with wire Connect beakers with salt bridge Spontaneous reaction Cr2O7−2 ( aq ) + 14 H + ( aq ) + 6 I − ( aq ) → 2Cr +3 ( aq ) + 3I 2 ( s ) + 7 H 2O( l ) Cell EMF The potential energy of the electrons is higher at the anode so they flow spontaneously through the external circuit to the cathode. The difference in potential energy is measured in volts A volt is one joule per coulomb of charge 1V = 1 J C Charge on one electron is 1.602 x 10-19 C 1Coulomb corresponds to the charge on 6.24 x 1018 electrons. Potential difference is called the electromotive force (EMF) or cell voltage 0 Zn( s ) + Cu +2 ( aq,1M ) → Zn +2 ( aq,1M ) + Cu( s ) Ecell = +1.10V Standard Reduction (Half-Cell) Potentials A→ B AB Ecell = E( B ) − E( A ) X→A XA = E( A ) − E( X ) Ecell X →B XB = E( B ) − E( X ) Ecell AB XB XA Ecell = Ecell ( B ) − Ecell ( A) X is the reference electrode XB XA Ecell ( B )& Ecell ( A) are called the standard reduction potentials XB 0 Ecell ( B ) = Ered (B) 0 XA Ecell ( A ) = Ered ( A) Reference Electrode is the Standard Hydrogen Electrode 0 2 H + ( aq,1M ) + 2e − → H 2 ( g ,1atm ) Ered = 0V EMF Example Determine the half-reactions that occur at the anode and cathode and the cell EMF for the half cells +3 Al( s ) / Al ( aq ) I 2 ( s ) / I −1( aq ) Cr2O7−2 ( aq ) / Cr +3 ( aq ) −1 I 2 ( s ) / I ( aq ) +2 Co ( aq ) / Co( s ) AgCl( s ) / Ag( s ) EMF, Free Energy & the Nernst Equation ∆G = −nFEcell F = the Faraday the charge on one mole of electrons (6.02214 x 10 23 e/mol)(1.60218 x 10 -19 C/e)=96500C/mol n = the number of electrons transferred in the reaction ∆G = ∆G + RT ln Q 0 −nFEcell = −nFE 0 cell Ecell = E 0 cell Ecell = E 0 cell + RT ln Q RT − ln Q nF 0.0592 − log Q n Example of Nernst Equation Zn( s ) + Cu +2 ( aq ) → Zn +2 ( aq ) + Cu( s ) Ecell 0.0592V [ Zn +2 ( aq )] log = 1.10V − 2 [ Cu +2 ( aq )] [ Cu +2 ] = 5.0 M , [ Zn +2 ] = 0.05M Ecell 0.0592V 0.05 = 1.10V − log 2 5 .0 Ecell 0.0592V = 1.10V − log 10−2 2 Ecell = 1.10V + 0.0592V = 1.16V ∆G Examples Given the reaction 3Ni +2 ( aq ) + 2Cr( OH )3 ( s ) + 10OH − ( aq ) → 3Ni( s ) + 2CrO4−2 ( aq ) + 8H 2O( l ) What is the value of n? What is ∆G 0 ? Same question for 1 2 Ag( s ) + O2 ( g ) + 2 H + ( aq ) → 2 Ag + ( aq ) + H 2O( l ) 2 Nernst Equation Example Zn( s ) + 2 H + ( aq ) → Zn +2 ( aq ) + H 2 ( g ) If the voltage in this cell is 0.45V at 250C when [Zn+2] =1.0M and PH2=1.0 atm, what is the concentration of H+? Concentration Cell Examples Two Hydrogen electrodes Electrode 1 PH 2 = 1.00atm, [H + (aq)]=? Electrode 2 PH 2 = 1.00atm, [H + (aq)]=1.00M T=298K Ecell = 0.211 V Current flow: electrode 1 to electrode 2 pH = ? Consider two Zn(s)-Zn+2(aq) half cells [ Zn +2 ( aq )]( cell 1 ) = 1.35M [ Zn +2 ( aq )]( cell 2 ) = 3.75 x10−4 M Which is anode? What is emf of cell? Strengths of Oxidizing/Reducing Agents Standard reduction potentials can be used to understand aqueous reaction chemistry 0 The more positive Ered the greater the tendency for the reactant of the half reactant to be reduced and, therefore, to oxidize another species. F2 ( g ) + 2e − → 2 F − ( aq ) 0 Ered = +2.87 V The half-reaction with the smallest 0 (algebraically) Ered is most easily reversed as an oxidation Li + ( aq ) + e − → Li( s ) 0 Ered = −3.05 V Rank the following ions in order of increasing strength as oxidizing agents NO3− ( aq ) Ag + ( aq ) Cr2O7-2 ( aq ) Rank the following ions in order of increasing strength as reducing agents I − ( aq ) Fe(s) Al(s) Batteries Portable, self-contained electrochemical power source that consists of one or more voltaic cells Primary Cell Cannot be recharged Secondary Cell Can be recharged Lead-Acid Battery Cathode: PbO2 (s)+HSO4− ( aq ) + 3H + ( aq ) + 2e− → 0 PbSO4 ( s ) + 2 H 2O( l ) Ered = +1.685 V Anode: Pb( s ) + HSO4- ( aq ) → PbSO4 ( s ) + H + ( aq ) + 2e − 0 = −0.356 V Ered Cell Reaction: PbO2 (s)+Pb(s)+2HSO4- ( aq ) + 2 H + ( aq ) → 2 PbSO4 ( s ) + 2 H 2O( l ) 0 Ecell = E 0 ( cathode ) − E 0 ( anode ) 0 Ecell = ( +1.685 V ) - (-0.356 V ) = + 2.041 V 12 V lead-acid battery consists of 6 voltaic cells and is rechargeable Alkaline Battery Cathode: 2MnO2 (s)+2H 2O(l)+2e→ 2 MnO( OH )( s ) + 2OH − ( aq ) Anode: Zn(s)+2OH - (aq) → Zn(OH)2 (s)+2e0 Ecell = 1.55V Fuel Cells Cathode : 0 O2 ( g ) + 2 H 2O( l ) + 4e − → 4OH − ( aq ) Ered = +0.40V Anode : 2 H 2 ( g ) + 4OH − ( aq ) → 4 H 2O( l ) + 4e− 0 Ered = −0.83V Re action : 0 2 H 2 ( g ) + 2O2 ( g ) → 2 H 2O( aq ) Ecell = +1.23V Batteries in series Corrosion Corrosion occurs when a metal undergoes a spontaneous redox reaction and is converted to an unwanted substance. Oxidation is usually the thermodynamically favored process for a metal Corrosion of Iron Preventing Iron from Corroding Cathodic Protection Electroplating Electrolysis with an active electrode Ni( s ) → Ni +2 ( aq ) + 2e − 0 Ered = −0.28V 2 H 2O( l ) → O2 ( g ) + 4 H + ( aq ) + 4e − Ni +2 ( aq ) + 2e − → Ni( s ) 0 Ered = +1.23V 0 Ered = −0.28V 0 2 H 2O( l ) + 2e − → H 2 ( g ) + 2OH − ( aq ) Ered = −0.83V Quantitative Aspects of Electrolysis Stoichiometry of a half-reaction shows how many electrons are needed for the electrolysis + − Na + e → Na Cu +2 + 2e − → Cu +3 − Al + 3e → Al Current measured in amperes (A) 1A = 1 Coulomb C =1 sec ond s Coulombs of charge=magnitude of current x duration of current Calculate the number of grams of Al produced in 1.00 hour by the electrolysis of molten AlCl3 if the current is 10.0A. The half-reaction for the formation of magnesium metal upon electrolysis of molten MgCl2 is Mg +2 + 2e − → Mg Calculate the mass of magnesium formed upon the passage of a current of 60.0A for 4.00x103 s Electrical Work Free energy change in a process is equal to the maximum useful work that can be extracted from the process. ∆G = wmax = − nFE wmax = nFEexternal > 0 The unit of electrical power is the Watt(W) Joule J 1Watt = 1 =1 sec ond s Unit of energy employed by utility companies is kilo-Watt-hour (kWh) 1kWh = 3.6 x10 J 6 Calculate the number of kilowatt-hours required to produce 1.0 x 103 kg of Al by electrolysis of Al+3 if the applied voltage is 5.50V w = nFE Cell EMF and Chemical Equilibrium ∆G = ∆G 0 + RT ln Q At equilibrium ∆G = 0 & Q = K eq 0 ∆G 0 = − RT ln K eq = −nFEcell 0 nFEcell ln K eq = RT 0 nEcell ; T=298K log K eq = 0.0592 Calculate the equilibrium constant for the oxidation of Fe+2 by O2 in an acidic solution Fe +2 ( aq ) + O2 ( g ) → Fe +3 ( aq ) + H 2O( l ) Fe +2 → Fe +3 + e − 0 Ered = +0.77V O2 ( g ) → 2 H 2O( l ) 4 H + ( aq ) + O2 ( g ) → 2 H 2O( l ) 0 4 H + ( aq ) + O2 ( g ) + 4e − → 2 H 2O( l ) Ered =+1.23V 0 n = 4 ; Ecell = +1.23V − 0.77V = 0.46V log K eq = 4 x 0.46V = 31 0.0592V K eq = 1.0 x1031