Flashlights and Simple Circuits
advertisement

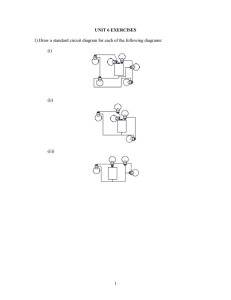
Activity #1: Exploring Flashlights and Open/Closed circuits What is a simple circuit?? A simple circuit consists of minimum things that are required for the functioning of a circuit. 1. A source of electrical energy (typically a battery or an electrical outlet) 2. A path for the movement of charges. (conductor - wire) 3. An electrical resistor which is basically any device/object that uses electricity to do work. (a light bulb, electric motor, heating element, speaker, etc.) In order for a circuit to function properly, the electricity must leave one end of the power source and return to the opposite end in an unbroken loop, or "circle." In the case of a battery, the electricity leaves the negative (-) end and returns to the positive (+) end. In a wall outlet, there is also a positive end and a negative end — the two holes into which the two prongs of a plug fit. In the early days of electricity, when Thomas Edison was busy working on his lightbulb, all circuits were simple and generally followed a straight-line path. These types of circuits are known as series circuits, and are the same systems used by most holiday tree lights. Many circuits can get really complicated. There are parallel, switched, integrated, and fused circuits. But no matter how you stack it, circuits are still a circle of electricity! Figures below show a simple circuit containing, one battery, two wires and a bulb. An electric current is a flow of microscopic particles called ELECTRONS flowing through wires and electronic components. It can be likened to the flow of water through pipes and radiators etc. As water is pushed through pipes by a pump, electric current is pushed through wires by a battery. Hot water does work by heating radiators. Electric current does work by heating fires, lighting lamps, ringing bells, electroplating etc. A basic law of the universe is that like charges repel and unlike attract. Two negatives will repel each other. A negative and a positive will attract each other. An electron has a negative charge. The negative (-ve) terminal of a battery will push negative electrons along a wire. The positive (+ve) terminal of a battery will attract negative electrons along a wire. Electrons will therefore flow from the -ve terminal of a battery, through the lamp, to the positive terminal. The flow of electricity is from the higher potential (+) terminal of the battery through the bulb and back to the negative (-) terminal in a continuous flow. Symbols for the battery, switch and the bulb or the resistor. Figure below shows a series circuit. The single bulb is glowing as the switch is closed. The two bulbs are glowing as the switch is closed. Neither of the bulbs are lit up as the circuit is broken. No Electricity can flow at all!! Schematic diagram of a simple circuit Bulb Battery I Bulb Bulb Battery Basics v History The first battery was demonstrated in 1800 by Count Alessandro Volta. His experiments showed that different metals in contact with each other could create electricity. He constructed a stack of discs of zinc alternating and blotting paper soaked in saltwater and silver or copper. When wires of different metals were attached to both the top and bottom discs, Volta was able to measure a voltage and a current. He also discovered that higher the pile, the higher the voltage. The current is produced because a chemical reaction arising from the different electron attracting capabilities of the two metals. The device became know as a “voltaic pile” (French word for battery is ‘pile’). Although they were large and bulky, voltaic piles provided the only practical source of electricity in the early 19th century. v How they work?? The pile or battery remained in the laboratory for years, until a newly invented telegraph and telephone created a demand for reliable electrical power. The “dry cell” battery was then invented in the 1860s for use with telegraph. The dry cell is not completely dry. The first battery was created by Alessandro Volta in 1800. To create his battery, he made a stack by alternating layers of zinc, blotting paper soaked in salt water, and silver. This arrangement was known as a voltaic pile. The top and bottom layers of the pile must be different metals. If you attach a wire to the top and bottom of the pile, you can measure a voltage and a current from the pile. The pile can be stacked as high as you like, and each layer will increase the voltage by a fixed amount. A dry cell produces about 1.5Volts. You can create your own voltaic pile using coins and paper towels. To do these experiments accurately, you will want to purchase an inexpensive ($10 to $20) volt-ohm meter at the local electronics or hardware store. Make sure that the meter can read low voltages(in the 1-volt range) and low currents (in the 5- to 10-milliamp range). This way, you will be able to see exactly what your battery is doing. Mix salt with water (as much salt as the water will hold) and soak the paper towel in this brine. Then create a pile by alternating pennies and nickels. See what kind of voltage and current the pile produces. Try a different number of layers and see what effect it has on voltage. Then try alternating pennies and dimes and see what happens. Also try dimes and nickels. Other metals to try include aluminum foil and steel. Each metallic combination should produce a slightly different voltage. v Flow of Electrons……. Electrochemical reactions take place and this moves electrons from one end of the battery to the other end of the battery. The bottom terminal is the positive terminal and the top terminal is the negative terminal. Free electrons at the negative terminal move towards the positive terminal. The flow of electrons is called the electric current. Electrons are the particles that carry the electric current. FlashLight Basics v History In the 1890s American Ever-Ready Company founder Conrad Hubert invented the electric hand torch. Hubert acquired the patent in 1898. They were made of paper and fiber tubes, with a bulb and a brass reflector. At that time batteries were weak and bulbs were still developing, so the first flashlights produced only a brief “Flash” of light – which gave the invention its name. v How do they work?? There are seven main components to a flashlight: è è Casing: Contacts: è è Switch: Reflector: è Lens: è Batteries: Holds all the components of the flashlight. Thin spring or strip of metal usually copper or brass that serves as the connection between the battery, lamp and switch. Could be in “on” or “off” position. Plastic coated with a reflective aluminum layer to help brighten the effective light of the bulb. Plastic cover in front of the bulb to protect the lamp which could be easily broken. Provide the power to the flashlight or provide the energy for the charges to move through the circuit.. When the switch is in the “on” position, it connects the two contract strips which allow electrons to flow. The batteries provide the power to the flashlight, and sit on top of a small spring that is connected to one of the contact strips. This contact strip connects the switch with the bulb. Finally, another contact connects the bulb to the top of the battery, completing the circuit.