Chapter 18 Heat and the First Law of Thermodynamics
advertisement
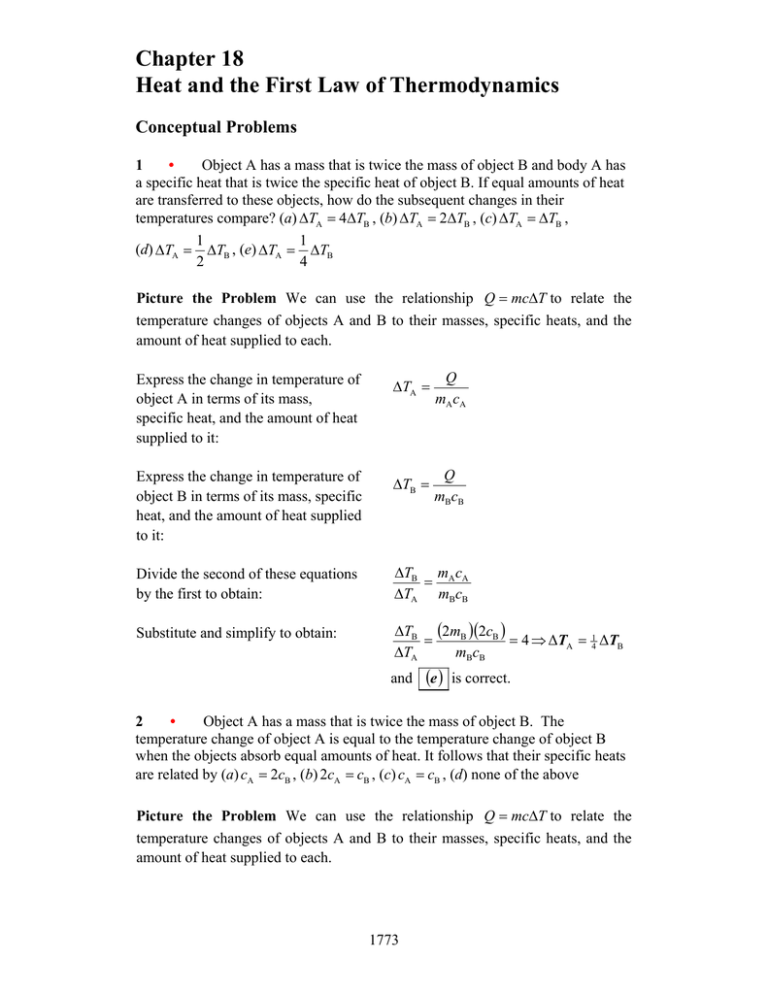
Chapter 18 Heat and the First Law of Thermodynamics Conceptual Problems 1 • Object A has a mass that is twice the mass of object B and body A has a specific heat that is twice the specific heat of object B. If equal amounts of heat are transferred to these objects, how do the subsequent changes in their temperatures compare? (a) ΔTA = 4ΔTB , (b) ΔTA = 2ΔTB , (c) ΔTA = ΔTB , 1 1 (d) ΔTA = ΔTB , (e) ΔTA = ΔTB 2 4 Picture the Problem We can use the relationship Q = mcΔT to relate the temperature changes of objects A and B to their masses, specific heats, and the amount of heat supplied to each. Express the change in temperature of object A in terms of its mass, specific heat, and the amount of heat supplied to it: ΔTA = Q mA cA Express the change in temperature of object B in terms of its mass, specific heat, and the amount of heat supplied to it: ΔTB = Q mBcB Divide the second of these equations by the first to obtain: ΔTB mA cA = ΔTA mBcB Substitute and simplify to obtain: ΔTB (2mB )(2cB ) = = 4 ⇒ ΔTA = 14 ΔTB mBcB ΔTA and (e ) is correct. 2 • Object A has a mass that is twice the mass of object B. The temperature change of object A is equal to the temperature change of object B when the objects absorb equal amounts of heat. It follows that their specific heats are related by (a) cA = 2cB , (b) 2cA = cB , (c) cA = cB , (d) none of the above Picture the Problem We can use the relationship Q = mcΔT to relate the temperature changes of objects A and B to their masses, specific heats, and the amount of heat supplied to each. 1773 1774 Chapter 18 Relate the temperature change of object A to its specific heat and mass: ΔTA = Q mA c A Relate the temperature change of object B to its specific heat and mass: ΔTB = Q mB c B Equate the temperature changes and simplify to obtain: 1 1 = mB c B mA c A Solve for cA: cA = mB m cB = B cB = 12 cB mA 2 mB and (b) is correct. 3 • [SSM] The specific heat of aluminum is more than twice the specific heat of copper. A block of copper and a block of aluminum have the same mass and temperature (20ºC). The blocks are simultaneously dropped into a single calorimeter containing water at 40ºC. Which statement is true when thermal equilibrium is reached? (a) The aluminum block is at a higher temperature than the copper block. (b) The aluminum block has absorbed less energy than the copper block. (c) The aluminum block has absorbed more energy than the copper block. (d) Both (a) and (c) are correct statements. Picture the Problem We can use the relationship Q = mcΔT to relate the amount of energy absorbed by the aluminum and copper blocks to their masses, specific heats, and temperature changes. Express the energy absorbed by the aluminum block: QAl = mAlcAl ΔT Express the energy absorbed by the copper block: QCu = mCu cCu ΔT Divide the second of these equations by the first to obtain: QCu mCu cCu ΔT = QAl mAlcAl ΔT Because the block’s masses are the same and they experience the same change in temperature: QCu cCu = <1 QAl cAl or QCu < QAl and (c ) is correct. Heat and the First Law of Thermodynamics 1775 4 • A block of copper is in a pot of boiling water and has a temperature of 100°C. The block is removed from the boiling water and immediately placed in an insulated container which has a quantity of water that has a temperature of 20°C and has the same mass as the block of copper. (The heat capacity of the insulated container is negligible.) The final temperature will be closest to (a) 40°C, (b) 60°C, (c) 80°C. Determine the Concept We can use the relationship Q = mcΔT to relate the temperature changes of the block of copper and the boiling water to their masses, specific heats, and the amount of heat supplied to or absorbed by each. Relate the heat supplied by the block of copper to its specific heat, mass, and temperature change as it cools to its equilibrium temperature: ΔQCu = mCu cCu ΔTCu Relate the heat absorbed by the water to its specific heat, mass, and temperature change as it warms to its equilibrium temperature: ΔQH2O = m H2O cH2O ΔTH2O = mCu cCu (Tequil − 100°C ) = m H2O cH2O (Tequil − 20°C ) Because thermal energy is conserved in this mixing process: ΔQ = ΔQCu + ΔQH2O = 0 or mCu cCu (Tequil − 100°C ) + mH2O cH2O (Tequil − 20°C ) = 0 Because the mass of the water is equal to the mass of the block of copper: cCu (Tequil − 100°C ) + cH2O (Tequil − 20°C ) = 0 Solving for Tequil yields: Tequil = (100°C)cCu + (20°C)cH O 2 cCu + cH 2 O Substitute numerical values (See Table 18-1 for the specific heats of copper and water.)and evaluate Tequil: Tequil = (100°C)(24.5 J/mol ⋅ K ) + (20°C)(75.2 J/mol ⋅ K ) = 40°C 24.5 J/mol ⋅ K + 75.2 J/mol ⋅ K and (b ) is correct. 1776 Chapter 18 5 • You pour both a certain amount of water at 100°C and an equal amount of water at room temperature (20°C) into an insulated container. The final temperature of the mixture will be (a) 60°C, (b) less than 60°C, (c) greater than 60°C. Determine the Concept We can use the relationship Q = mcΔT to relate the temperature changes of the room temperature water and the boiling water to their masses, specific heats, and the amount of heat supplied to or absorbed by each. Relate the heat supplied by the water to its specific heat, mass, and temperature change as it cools to its equilibrium temperature: ΔQhot H2O = m hot H2O c boiling H2O ΔThot H2O Relate the heat absorbed by the water to its specific heat, mass, and temperature change as it warms to its equilibrium temperature: ΔQH2O = m H2O cH2O ΔTH2O = m hot H2O c hot H2O (Tequil − 100°C ) = m H2O cH2O (Tequil − 20°C ) Because thermal energy is conserved in this mixing process: ΔQ = ΔQhot H2O + ΔQH2O = 0 or m hot H2O chot H2O (Tequil − 100°C ) + m H2O cH2O (Tequil − 20°C ) = 0 Because the mass of the boiling water is equal to the mass of the water that is initially at 20°C and the specific heat of water is independent of its temperature: Tequil − 100°C + Tequil − 20°C = 0 Solving for Tequil yields: Tequil = 60°C and (a ) is correct. 6 • You pour some water at 100°C and some ice cubes at 0°C into an insulated container. The final temperature of the mixture will be (a) 50°C, (b) less than 50°C but larger than 0°C, (c) 0°C, (d) You cannot tell the final temperature from the data given. Determine the Concept We would need to know the mass of the boiling water and the mass of the melting ice in order to determine the final temperature of the mixture. (d ) is correct. Heat and the First Law of Thermodynamics 1777 7 • You pour water at 100oC and some ice cubes at 0°C into an insulated container. When thermal equilibrium is reached, you notice some ice remains and floats in liquid water. The final temperature of the mixture is (a) above 0°C, (b) less than 0°C, (c) 0°C, (d) You cannot tell the final temperature from the data given Determine the Concept Because some ice remains when thermal equilibrium is reached, the equilibrium temperature must be the temperature of the ice cubes at their melting point. (c ) is correct. 8 • Joule’s experiment establishing the mechanical equivalence of heat involved the conversion of mechanical energy into internal energy. Give some everyday examples in which some of the internal energy of a system is converted into mechanical energy. Determine the Concept One example from everyday life is that of a hot gas that expands and does work−as in the piston of an engine. Another example is the gas escaping from a can of spray paint. As it escapes, it moves air molecules and, in the process, does work against atmospheric pressure. 9 • Can a gas absorb heat while its internal energy does not change? If so, give an example. If not, explain why not. Determine the Concept Yes, if the absorbed heat is converted into mechanical work. Providing this condition is satisfied, no energy goes into changing the internal energy of the gas and its temperature will remain constant. 10 • The equation ΔEint = Q + W is the formal statement of the first law of thermodynamics. In this equation, the quantities Q and W, respectively, represent (a) the heat absorbed by the system and the work done by the system, (b) the heat absorbed by the system and the work done on the system, (c) the heat released by the system and the work done by the system, (d) the heat released by the system and the work done on the system. Determine the Concept According to the first law of thermodynamics, the change in the internal energy of the system is equal to the heat that enters the system plus the work done on the system. (b) is correct. 11 • [SSM] A real gas cools during a free expansion, while an ideal gas does not cool during a free expansion. Explain the reason for this difference. Determine the Concept Particles that attract each other have more potential energy the farther apart they are. In a real gas the molecules exert weak attractive forces on each other. These forces increase the internal potential energy during an 1778 Chapter 18 expansion. An increase in potential energy means a decrease in kinetic energy, and a decrease in kinetic energy means a decrease in translational kinetic energy. Thus, there is a decrease in temperature. 12 • An ideal gas that has a pressure of 1.0 atm and a temperature of 300 K is confined to half of an insulated container by a thin partition. The other half of the container is a vacuum. The partition is punctured and equilibrium is quickly reestablished. Which of the following is correct? (a) The gas pressure is 0.50 atm and the temperature of the gas is 150 K. (b) The gas pressure is 1.0 atm and the temperature of the gas is 150 K. (c) The gas pressure is 0.50 atm and the temperature of the gas is 300 K. (d) None of the above. Determine the Concept Because the container is insulated, no energy is exchanged with the surroundings during the expansion of the gas. Neither is any work done on or by the gas during this process. Hence, the internal energy of the gas does not change and we can conclude that the equilibrium temperature will be the same as the initial temperature. Applying the ideal-gas law for a fixed amount of gas we see that the pressure at equilibrium must be half an atmosphere. (c ) is correct. 13 • A gas consists of ions that repel each other. The gas undergoes a free expansion in which no heat is absorbed or released and no work is done. Does the temperature of the gas increase, decrease, or remain the same? Explain your answer. Determine the Concept Particles that repel each other have more potential energy the closer together they are. The repulsive forces decrease the internal potential energy during an expansion. A decrease in potential energy means an increase in kinetic energy, and an increase in kinetic energy means an increase in translational kinetic energy. Thus, there is an increase in temperature. 14 • Two gas-filled rubber balloons that have equal volumes are located at the bottom of a dark, cold lake. The temperature of the water decreases with increasing depth. One balloon rises rapidly and expands adiabatically as it rises. The other balloon rises more slowly and expands isothermally. The pressure in each balloon remains equal to the pressure in the water just next to the balloon. Which balloon has the larger volume when it reaches the surface of the lake? Explain your answer. Determine the Concept The balloon that expands isothermally is larger when it reaches the surface. The balloon that expands adiabatically will be at a lower temperature than the one that expands isothermally. Because each balloon has the Heat and the First Law of Thermodynamics 1779 same number of gas molecules and are at the same pressure, the one with the higher temperature will be bigger. An analytical argument that leads to the same conclusion is shown below. 1γ Letting the subscript ″a″ denote the adiabatic process and the subscript ″i″ denote the isothermal process, express the equation of state for the adiabatic balloon: ⎛P ⎞ P0V0 = Pf Vf,a ⇒ Vf,a = V0 ⎜⎜ 0 ⎟⎟ ⎝ Pf ⎠ For the isothermal balloon: ⎛P ⎞ P0V0 = Pf Vf,i ⇒ Vf,i = V0 ⎜⎜ 0 ⎟⎟ ⎝ Pf ⎠ Divide the second of these equations by the first and simplify to obtain: ⎛P ⎞ V0 ⎜⎜ 0 ⎟⎟ 1−1 λ Vf,i Pf ⎠ ⎛ P0 ⎞ ⎝ = ⎜⎜ ⎟⎟ = 1γ Vf,a ⎛ P0 ⎞ ⎝ Pf ⎠ V0 ⎜⎜ ⎟⎟ ⎝ Pf ⎠ Because P0/Pf > 1 and γ > 1: γ γ Vf,i > Vf,a 15 • A gas changes its state quasi-statically from A to C along the paths shown in Figure 18-21. The work done by the gas is (a) greatest for path A→B→C, (b) least for path A→C, (c) greatest for path A→D→C, (d) The same for all three paths. Determine the Concept The work done along each of these paths equals the area under its curve. The area is greatest for the path A→B→C and least for the path A→D→C. (a ) is correct. 16 • When an ideal gas undergoes an adiabatic process, (a) no work is done by the system, (b) no heat is transferred to the system, (c) the internal energy of the system remains constant, (d) the amount of heat transferred into the system equals the amount of work done by the system. Determine the Concept An adiabatic process is, by definition, one for which no heat enters or leaves the system. (b) is correct. 1780 Chapter 18 True or false: 17 • (a) When a system can go from state 1 to state 2 by several different processes, the amount of heat absorbed by the system will be the same for all processes. When a system can go from state 1 to state 2 by several different processes, the work done on the system will be the same for all processes. When a system can go from state 1 to state 2 by several different processes, the change in the internal energy of the system will be the same for all processes. The internal energy of a given amount of an ideal gas depends only on its absolute temperature. A quasi-static process is one in which the system is never far from being in equilibrium. For any substance that expands when heated, its Cp is greater than its Cv. (b) (c) (d) (e) (f) (a) False. The amount the internal energy of the system changes along any path depends only on the temperatures at state 1 and state 2, but because ΔEint = Qin + Won , Qin depends on Won, which, in turn, depends on the path taken from state 1 to state 2. (b) False. The work done on the system depends on the path taken from state 1 to state 2. (c) True. The amount the internal energy of the system changes along any path depends only on the temperatures at state 1 and state 2. (d) True. For an ideal gas, Eint = 32 nRT . For a ″given amount″ of an ideal gas, n is constant. (e) True. This is the definition of a quasi-static process. (f) True. All materials have values for Cp and Cv for which it is true that Cp is greater than Cv. For liquids and solids we generally ignore the very small difference between Cp and Cv . 18 • The volume of a sample of gas remains constant while its pressure increases. (a) The internal energy of the system is unchanged. (b) The system does work. (c) The system absorbs no heat. (d) The change in internal energy must equal the heat absorbed by the system. (e) None of the above. Determine the Concept For a constant-volume process, no work is done on or by the gas. Applying the first law of thermodynamics, we obtain Qin = ΔEint. Because the temperature must change during such a process, we can conclude that Heat and the First Law of Thermodynamics 1781 ΔEint ≠ 0 and hence Qin ≠ 0. (d ) is correct. 19 • When an ideal gas undergoes an isothermal process, (a) no work is done by the system, (b) no heat is absorbed by the system, (c) the heat absorbed by the system equals the change in the system’s internal energy, (d) the heat absorbed by the system equals the work done by the system. Determine the Concept Because the temperature does not change during an isothermal process, the change in the internal energy of the gas is zero. Applying the first law of thermodynamics, we obtain Qin = −Won = Wby the system. Hence (d ) is correct. 20 •• Consider the following series of sequential quasi-static processes that a system undergoes: (1) an adiabatic expansion, (2) an isothermal expansion, (3) an adiabatic compression and (4) an isothermal compression which brings the system back to its original state. Sketch the series of processes on a PV diagram, and then sketch the series of processes on a VT diagram (in which volume is plotted as function of temperature). Determine the Concept In the graphs shown below, ″I″ denotes an isothermal process, ″A″ denotes an adiabatic process, ″E″ denotes an expansion, and ″C″ denotes a compression. P V AE IC IE AE IC AC AC IE V T 21 •• [SSM] An ideal gas in a cylinder is at pressure P and volume V. During a quasi-static adiabatic process, the gas is compressed until its volume has decreased to V/2. Then, in a quasi-static isothermal process, the gas is allowed to expand until its volume again has a value of V. What kind of process will return the system to its original state? Sketch the cycle on a graph. 1782 Chapter 18 Determine the Concept Adiabatic processes are steeper than isothermal processes. During an adiabatic process the pressure varies as V − γ , whereas during an isothermal processes the pressure varies as V −1 . Thus when a gas expands isothermally, the pressure doesn’t quite go down as far as the starting point. Thus the final process necessary is a constant-volume process dropping back to the initial pressure. Heat must be removed from the system in order for this to occur. P isothermal adiabatic constant volume Vf = 12 Vi Vi V 22 •• Metal A is denser than metal B. Which would you expect to have a higher heat capacity per unit mass, metal A or metal B? Why? Determine the Concept We can use the definition of heat capacity, the DulongPetit law, and the relationship between the mass of a substance and its molar mass to predict whether metal A or metal B has a higher heat capacity per unit mass. The specific heat of metal A is given by: From the Dulong-Petit law, the molar heat capacity of most metals is approximately: Substituting for c′ yields: Similarly, the specific heat of metal B is given by: Dividing cA by cB and simplifying yields: Because metal A is denser than metal B: cA = c' MA c' = 3 R cA = 3R MA cB = 3R MB 3R cA M A M B M = = ⇒ cA = B cB 3R cB MA MA MB cA > cB Heat and the First Law of Thermodynamics 1783 23 •• An ideal gas undergoes a process during which P V = constant and the volume of the gas decreases. Does its temperature increase, decrease, or remain the same during this process? Explain. Picture the Problem We can use the given dependence of the pressure on the volume and the ideal-gas law to show that if the volume decreases, so does the temperature. We’re given that: P V = constant Because the gas is an ideal gas: PV = P V ( ) V = constant × V = nRT Solving for T yields: T= (constant ) nR V ∝ V Because T varies with the square root of V, if the volume decreases, the temperature decreases. Estimation and Approximation 24 • During the early stages of designing a modern electric generating plant, you are in charge of the team of environmental engineers. The new plant is to be located on the ocean and will use ocean water for cooling. The plant will produce electrical power at the rate of 1.00 GW. Because the plant will have an efficiency of one-third (typical of most modern plants), heat will be released to the cooling water at the rate of 2.00 GW. If environmental codes require that only water with a temperature increase of 15°F or less can be returned to the ocean, estimate the flow rate (in kg/s) of cooling water through the plant. Picture the Problem The rate at which thermal energy is delivered to the ocean by the power plant is given by P = ΔQ Δt where Q = mcΔT Express the rate at which thermal energy is delivered to the ocean: Our interest is in the flow rate of the cooling water. Solving for Δm Δt yields: P= ΔQ Δ Δm = (mcΔT ) = cΔT Δt Δt Δt P Δm = Δt cΔT 1784 Chapter 18 Substitute numerical values and evaluate Δm Δt : Δm 2.00 × 109 J/s = Δt ⎛ kJ ⎞ ⎛ 5 C° ⎞ ⎜⎜ 4.184 ⎟⎟ ⎜15 F° × ⎟ kg ⋅ C° ⎠ ⎝ 9 F° ⎠ ⎝ = 5.7 ×10 4 kg/s 25 •• [SSM] A ″typical″ microwave oven has a power consumption of about 1200 W. Estimate how long it should take to boil a cup of water in the microwave assuming that 50% of the electrical power consumption goes into heating the water. How does this estimate correspond to everyday experience? Picture the Problem Assume that the water is initially at 30°C and that the cup contains 200 g of water. We can use the definition of power to express the required time to bring the water to a boil in terms of its mass, heat capacity, change in temperature, and the rate at which energy is supplied to the water by the microwave oven. Use the definition of power to relate the energy needed to warm the water to the elapsed time: P= ΔW mcΔT mcΔT = ⇒ Δt = Δt P Δt Substitute numerical values and evaluate Δt: kJ ⎞ ⎟ (100°C − 30°C ) kg ⋅ K ⎟⎠ ⎝ Δt = = 97.63 s ≈ 1.6 min , 600 W an elapsed time that seems to be consistent with experience. (0.200 kg )⎛⎜⎜ 4.184 26 •• A demonstration of the heating of a gas under adiabatic compression involves putting a small strip of paper into a large glass test tube, which is then sealed off with a piston. If the piston compresses the trapped air very rapidly, the paper will catch fire. Assuming that the burning point of paper is 451ºF, estimate the factor by which the volume of the air trapped by the piston must be reduced for this demonstration to work. Picture the Problem The adiabatic compression from an initial volume V1 to a final volume V2 between the isotherms at temperatures T1 and T2 is shown to the right. We’ll assume a room temperature of 300 K and apply the equation for a quasi-static adiabatic compression to 451ºF (506 K) with γair = 1.4 to solve for the ratio of the initial to the final volume of the air. Heat and the First Law of Thermodynamics 1785 P T2 = 506 K T1 = 300 K V2 Express TV γ −1 = constant in terms of the initial and final values of T and V: Substitute numerical values and evaluate V1/V2: V V1 1 T1V1γ −1 V ⎛ T ⎞ γ −1 = T2V2γ −1 ⇒ 1 = ⎜⎜ 2 ⎟⎟ V2 ⎝ T1 ⎠ V1 ⎛ 506 K ⎞ ⎟ =⎜ V2 ⎜⎝ 300 K ⎟⎠ 1 1.4−1 = 3.69 27 •• A small change in the volume of a liquid occurs when heating the liquid at constant pressure. Use the following data to estimate the contribution this fractional change makes to the heat capacity of water between 4ºC and 100ºC. The density of water at 4.00ºC and 1.00 atm pressure is 1.000 g/cm3. The density of liquid water at 100ºC and 1.00 atm pressure is 0.9584 g/cm3. Picture the Problem The heat capacity Cp of a sample of water is equal to the mass m of the sample times the specific heat capacity cp of water, where cp = 4186 J/kg·K. When the sample is heated, its temperature and its volume increases. The increase in temperature reflects the increase in the internal energy of the sample, and the increase in volume reflects the amount of work Won done by the sample on its surroundings. That is, Won = PΔV, where P is the pressure and ΔV is the change in volume of the sample. We are looking for the ratio of the work done by the sample to the heat absorbed by the sample. The heat that enters the water during the constant pressure process is given by: Qin = mcp ΔT The work that is done by the sample during this process is given by: Wby = PΔV = P(V100°C − V4.00°C ) 1786 Chapter 18 The volumes of the sample at these temperatures is related to its densities at these temperatures: ⎛ m m ⎞ ⎟⎟ Wby = P⎜⎜ − ⎝ ρ100°C ρ 4.00°C ⎠ ⎛ 1 1 ⎞ ⎟⎟ = Pm⎜⎜ − ρ ρ 4.00° C ⎠ ⎝ 100°C where m is the mass of the sample. Taking the ratio of Wby to Qin yields: Wby Qin = 1 ⎞ Pm ⎛ 1 ⎜⎜ ⎟ − mcp ΔT ⎝ ρ100°C ρ 4.00°C ⎟⎠ = 1 ⎞ P ⎛ 1 ⎜⎜ ⎟ − cp ΔT ⎝ ρ100°C ρ 4.00°C ⎟⎠ Substitute numerical values and evaluate Wby/Qin: ⎛ 101.325 × 103 Pa ⎜ 1 1 = − ⎜ kg kg Qin ⎛ J ⎞ ⎜ ⎜ 4186 kg ⋅ K ⎟ ( 96 K ) ⎝ 954.3 m3 1000 m3 ⎝ ⎠ Wby ⎞ ⎟ −3 ⎟ = 1.1×10 % ⎟ ⎠ Heat Capacity, Specific Heat, Latent Heat 28 • You designed a solar home that contains 1.00 × 105 kg of concrete (specific heat = 1.00 kJ/kg⋅K). How much heat is released by the concrete at night when it cools from 25.0ºC to 20.0ºC? Picture the Problem We can use the relationship Q = mcΔT to calculate the amount of heat given off by the concrete as it cools from 25.0°C to 20.0°C. Relate the heat given off by the concrete to its mass, specific heat, and change in temperature: Q = mcΔT Substitute numerical values and evaluate Q: ⎛ kJ ⎞ ⎟ (25.0°C − 20.0°C ) = 500 MJ Q = 1.00 × 105 kg ⎜⎜1.00 kg ⋅ K ⎟⎠ ⎝ ( ) 29 • [SSM] How much heat must be absorbed by 60.0 g of ice at –10.0ºC to transform it into 60.0 g of water at 40.0ºC? Picture the Problem We can find the amount of heat that must be absorbed by adding the heat required to warm the ice from −10.0°C to 0°C, the heat required to Heat and the First Law of Thermodynamics 1787 melt the ice, and the heat required to warm the water formed from the ice to 40.0°C. Express the total heat required: Q = Qwarm ice + Qmelt ice + Qwarm water Substitute for each term to obtain: Q = mcice ΔTice + mLf + mcwater ΔTwater = m(cice ΔTice + Lf + cwater ΔTwater ) Substitute numerical values (See Tables 18-1 and 18-2) and evaluate Q: ⎡⎛ kJ ⎞ kJ ⎟⎟ (0°C − (− 10.0°C )) + 333.5 Q = (0.0600 kg ) ⎢⎜⎜ 2.05 kg ⋅ K ⎠ kg ⎣⎝ ⎤ ⎛ kJ ⎞ ⎟⎟ (40.0°C − 0°C )⎥ + ⎜⎜ 4.184 kg ⋅ K ⎠ ⎝ ⎦ = 31.3 kJ 30 •• How much heat must be released by 0.100 kg of steam at 150ºC to transform it into 0.100 kg of ice at 0ºC? Picture the Problem We can find the amount of heat that must be removed by adding the heat that must be removed to cool the steam from 150°C to 100°C, the heat that must be removed to condense the steam to water, the heat that must be removed to cool the water from 100°C to 0°C, and the heat that must be removed to freeze the water. Express the total heat that must be removed: Q = Qcool steam + Qcondense steam + Qcool water + Qfreeze water Substitute for each term and simplify to obtain: Q = mcsteam ΔTsteam + mLv + mcwater ΔTwater + mLf = m(csteam ΔTsteam + Lv + cwater ΔTwater + Lf ) 1788 Chapter 18 Substitute numerical values (See Tables 18-1 and 18-2) and evaluate Q: ⎡⎛ kJ ⎞ MJ ⎟⎟ (150°C − 100°C ) + 2.26 Q = (0.100 kg ) ⎢⎜⎜ 2.02 kg ⋅ K ⎠ kg ⎣⎝ ⎛ kJ ⎞ kJ ⎤ ⎟⎟ (100°C − 0°C ) + 333.5 ⎥ + ⎜⎜ 4.184 kg ⋅ K ⎠ kg ⎦ ⎝ = 311 kJ 31 •• A 50.0-g piece of aluminum at 20ºC is cooled to –196ºC by placing it in a large container of liquid nitrogen at that temperature. How much nitrogen is vaporized? (Assume that the specific heat of aluminum is constant over this temperature range.) Picture the Problem We can find the amount of nitrogen vaporized by equating the heat gained by the liquid nitrogen and the heat lost by the piece of aluminum. Express the heat gained by the liquid nitrogen as it cools the piece of aluminum: QN = mN Lv, N Express the heat lost by the piece of aluminum as it cools: QAl = mAlcAl ΔTAl Equate these two expressions and solve for mN: mN Lv, N = mAl cAl ΔTAl and mN = mAl cAl ΔTAl Lv, N Substitute numerical values (see Table 18-1 for the specific heat of aluminum) and evaluate mN: mN = (0.0500 kg )⎛⎜⎜ 0.900 ⎝ kJ ⎞ ⎟ (20°C − (− 196°C )) kg ⋅ K ⎟⎠ = 48.8 g kJ 199 kg 32 •• You are supervising the creation of some lead castings for use in the construction industry. Each casting involves one of your workers pouring 0.500 kg of molten lead that has a temperature of 327ºC into a cavity in a large block of ice at 0ºC. How much liquid water should you plan on draining per hour if there Heat and the First Law of Thermodynamics 1789 are 100 workers who are able to each average one casting every 10.0 min? Picture the Problem Because the heat lost by the lead as it cools is gained by the block of ice (we’re assuming no heat is lost to the surroundings), we can apply the conservation of energy to determine how much ice melts. ΔQ = ΔQPb + ΔQW = 0 Apply the conservation of energy to this process: or − mPb (Lf, Pb + cPb ΔTPb ) + mw, 1 casting Lf, w = 0 Solving for mw yields: mW, 1 casting = Because there are N workers each turning out n castings per hour: mPb (Lf, Pb + cPb ΔTPb ) Lf, W m = nNmW.1 casting = nNmPb (Lf, Pb + cPb ΔTPb ) Lf, W Substitute numerical values and evaluate mw: ⎛ m= (100)(6)(0.500 kg )⎜⎜ 24.7 kJ + ⎛⎜⎜ 0.128 ⎝ kg ⎝ kJ 333.5 kg ⎞ kJ ⎞ ⎟⎟ (327°C − 0°C )⎟⎟ kg ⋅ K ⎠ ⎠ ≈ 60 kg Calorimetry 33 • [SSM] While spending the summer on your uncle’s horse farm, you spend a week apprenticing with his farrier (a person who makes and fits horseshoes). You observe the way he cools a shoe after pounding the hot, pliable shoe into the correct size and shape. Suppose a 750-g iron horseshoe is taken from the farrier’s fire, shaped, and at a temperature of 650°C, dropped into a 25.0-L bucket of water at 10.0°C. What is the final temperature of the water after the horseshoe and water arrive at equilibrium? Neglect any heating of the bucket and assume the specific heat of iron is 460 J/(kg ⋅ K) . Picture the Problem During this process the water will gain energy at the expense of the horseshoe. We can use conservation of energy to find the equilibrium temperature. See Table 18-1 for the specific heat of water. 1790 Chapter 18 Apply conservation of energy to obtain: ∑Q i = Qwarm the water + Qcool the horseshoe = 0 i or mwater c water (t f − 10.0°C ) + mFe cFe (t f − 650°C ) = 0 Solve for tf to obtain: tf = mwater c water (10.0°C ) + mFe cFe (650°C ) mwater c water + mFe cFe Substitute numerical values and evaluate tf: tf = (25.0 kg )⎛⎜⎜ 4.184 ⎛ kJ ⎞ kJ ⎞ ⎟⎟ (10.0°C ) + (0.750 kg )⎜⎜ 0.460 ⎟ (650°C ) kg ⋅ K ⎠ kg ⋅ K ⎟⎠ ⎝ ⎝ (25.0 kg )⎛⎜⎜ 4.184 kJ ⎞⎟⎟ + (0.750 kg )⎛⎜⎜ 0.460 kJ ⎞⎟⎟ kg ⋅ K ⎠ kg ⋅ K ⎠ ⎝ ⎝ = 12.1°C 34 • The specific heat of a certain metal can be determined by measuring the temperature change that occurs when a piece of the metal is heated and then placed in an insulated container that is made of the same material and contains water. Suppose the piece of metal has a mass of 100 g and is initially at 100ºC. The container has a mass of 200 g and contains 500 g of water at an initial temperature of 20.0ºC. The final temperature is 21.4ºC. What is the specific heat of the metal? Picture the Problem During this process the water and the container will gain thermal energy at the expense of the piece of metal. Applying conservation of energy will lead to an expression you can solve for the specific heat of the metal. Apply conservation of energy to obtain: ∑Q i = Qwarm the water + Qwarm the container + Qcool the metal sample = 0 i or mw c w ΔTw + mcontainer cmetal ΔTw + mmetal cmetal ΔTmetal = 0 Solving for cmetal yields: cmetal = mw c w ΔTw mmetal ΔTmetal − mcontainer ΔTw Heat and the First Law of Thermodynamics 1791 Substitute numerical values and evaluate cmetal : (0.500 kg )⎛⎜⎜ 4.184 cmetal kJ ⎞ ⎟ (21.4°C − 20.0°C ) kg ⋅ K ⎟⎠ ⎝ = (0.100 kg )(100°C − 21.4°C) − (0.200 kg )(21.4°C − 20.0°C) = 0.39 kJ kg ⋅ K Remarks: Consulting Table 18-1, we note that the metal is copper. 35 •• During his many appearances at the Tour de France, champion bicyclist Lance Armstrong typically expended an average power of 400 W, 5.0 hours a day for 20 days. What quantity of water, initially at 24ºC, could be brought to a boil if you could harness all of that energy? Picture the Problem We can use Q = mcΔT to express the mass m of water that can be heated through a temperature interval ΔT by an amount of heat energy Q. We can then find the amount of heat energy expended by Armstrong from the definition of power. Express the amount of heat energy Q required to raise the temperature of a mass m of water by ΔT: Use the definition of power to relate the heat energy expended by Armstrong to the rate at which he expended the energy: Substitute for Q to obtain: Substitute numerical values and evaluate m: Q = mcΔT ⇒ m = Q cΔT P= Q ⇒ Q = PΔt Δt m= PΔt cΔT h J 3600 s ⎞ ⎛ ⎛ ⎞ ⎜ 400 × ⎟ ⎜ 5.0 × 20 d ⎟ d s h ⎠⎝ ⎠ m=⎝ ⎛ kJ ⎞ ⎟ (100°C − 24°C ) ⎜⎜ 4.184 kg ⋅ K ⎟⎠ ⎝ = 4.5 × 10 2 kg 36 •• A 25.0-g glass tumbler contains 200 mL of water at 24.0ºC. If two 15.0-g ice cubes, each at a temperature of –3.00ºC, are dropped into the tumbler, what is the final temperature of the drink? Neglect any heat transfer between the tumbler and the room. 1792 Chapter 18 Picture the Problem First you need to convince yourself that there is enough energy in the water to melt all the ice. Once you’ve done that you can use conservation of energy to find the final equilibrium temperature. See Tables 18-1 and 18-2 for specific heats and the latent heat of fusion of water. First, determine the energy required to warm and melt all the ice: Qneeded = Qwarm ice + Qmelt ice = miceciceΔTwarm ice + mice Lf ⎛ kJ ⎞ ⎟ (3.00 C°) + (0.0300 kg )(333.5 kJ/kg ) = (0.0300 kg ) ⎜⎜ 2.05 kg ⋅ K ⎟⎠ ⎝ = 0.1845 kJ + 10.01 kJ = 10.19 kJ The maximum amount of energy available from the water and the tumbler is: Qavailable, max = m tumbler cglassΔTmax, tumbler + m water c water ΔTmax, water = m tumbler cglassΔTmax, tumbler + ρ waterVwater c water ΔTmax, water ⎛ kJ ⎞ ⎟ (0°C − 24.0°C ) = (0.0250 kg )⎜⎜ 0.840 kg ⋅ K ⎟⎠ ⎝ 10 −3 m 3 ⎞⎛ kJ ⎞ ⎛ 3 kg ⎞ ⎛ −3 ⎟⎟⎜⎜ 4.184 ⎟ + ⎜1.00 × 10 3 ⎟ ⎜⎜ 200 × 10 L × m ⎠⎝ L ⎠⎝ kg ⋅ K ⎟⎠ ⎝ × (0°C − 24.0°C ) = −0.5040 kJ − 20.08 kJ = −20.59 kJ Because Qavailable, max > Qneeded , all the ice will melt and the final temperature will be greater than 0°C. Apply conservation of energy to obtain: ∑Q = Q i cool the tumbler + Qcool the warm water + Qwarm the ice + Qmelt all the ice i + Qwarm the ice water = 0 or mtumblercglassΔTtumbler + mwater cwater ΔTwater + miceciceΔTwarn ice + mice Lf, ice + micecwater ΔTice water = 0 Heat and the First Law of Thermodynamics 1793 Substituting numerical values yields: (0.0250 kg )⎛⎜⎜ 0.840 ⎝ ⎛ kJ ⎞ kJ ⎞ ⎟⎟ (t f − 24.0°C ) + (0.200 kg )⎜⎜ 4.184 ⎟ (t f − 24.0°C ) kg ⋅ K ⎠ kg ⋅ K ⎟⎠ ⎝ ⎛ kJ ⎞ ⎟ (t f − 0°C ) = 0 + 0.1845 kJ + 10.01 kJ + (0.0300 kg )⎜⎜ 4.184 kg ⋅ K ⎟⎠ ⎝ Solving for tf yields: t f = 10.6°C 37 •• [SSM] A 200-g piece of ice at 0ºC is placed in 500 g of water at 20ºC. This system is in a container of negligible heat capacity and is insulated from its surroundings. (a) What is the final equilibrium temperature of the system? (b) How much of the ice melts? Picture the Problem Because we can not tell, without performing a couple of calculations, whether there is enough heat available in the 500 g of water to melt all of the ice, we’ll need to resolve this question first. See Tables 18-1 and 18-2 for specific heats and the latent heat of fusion of water. (a) Determine the energy required to melt 200 g of ice: ⎛ kJ ⎞ Qmelt ice = mice Lf = (0.200 kg )⎜⎜ 333.5 ⎟⎟ kg ⎠ ⎝ = 66.70 kJ The energy available from 500 g of water at 20ºC is: ⎛ kJ ⎞ ⎟⎟ (0°C − 20°C ) Qavailable, max = m water cwater ΔTwater = (0.500 kg )⎜⎜ 4.184 kg K ⋅ ⎠ ⎝ = −41.84 kJ Because Qavailable, max < Qmelt ice : (b) Equate the energy available from the water Qavailable, max to miceLf and The final temperature is 0°C. mice = Qavailable, max Lf solve for mice to obtain: Substitute numerical values and evaluate mice: mice = 41.84 kJ = 125 g kJ 333.5 kg 1794 Chapter 18 38 •• A 3.5-kg block of copper at a temperature of 80ºC is dropped into a bucket containing a mixture of ice and water whose total mass is 1.2 kg. When thermal equilibrium is reached, the temperature of the water is 8.0ºC. How much ice was in the bucket before the copper block was placed in it? (Assume that the heat capacity of the bucket is negligible.) Picture the Problem Because the bucket contains a mixture of ice and water initially, we know that its temperature must be 0°C. We can apply conservation of energy to find the amount of ice initially in the bucket. See Tables 18-1 and 18-2 for specific heats and the heats of fusion and vaporization of water. Apply conservation of energy to obtain: ∑Q i = Qmelt all + Qwarm the + Qcool the the ice i ice water Substitute for Qmelt all , Qwarm the , and Qcool the the ice ice water =0 copper block to obtain: copper block mice Lf + mice water c water ΔTice water + mCu cCu ΔTCu = 0 Solving for mice yields: mice = mCu cCu ΔTCu − mice water cwater ΔTice water Lf Substitute numerical values and evaluate mice: mice = (3.5 kg )⎛⎜⎜ 0.386 kJ ⎞ ⎟ (80°C − 8.0°C ) kg ⋅ K ⎟⎠ kJ 333.5 kg ⎝ − (1.2 kg )⎛⎜⎜ 4.184 ⎝ kJ ⎞ ⎟ (8.0°C − 0°C ) kg ⋅ K ⎟⎠ kJ 333.5 kg = 0.17 kg 39 •• A well-insulated bucket of negligible heat capacity contains 150 g of ice at 0ºC. (a) If 20 g of steam at 100ºC is injected into the bucket, what is the final equilibrium temperature of the system? (b) Is any ice left after the system reaches equilibrium? Heat and the First Law of Thermodynamics 1795 Picture the Problem First you need to convince yourself that there is enough energy in the steam to melt all the ice. Once you’ve done that you can use conservation of energy to find the final equilibrium temperature. See Tables 18-1 and 18-2 for specific heats and the heats of fusion and vaporization of water. Qmelt ice = mice Lf (a) First, determine the energy required to melt all the ice: = (0.150 kg )(333.5 kJ/kg ) = 50.03 kJ Find the maximum amount of energy available from the steam is: Qsteam, max = msteam Lv + msteam water c water ΔTmax ⎛ ⎛ kJ ⎞ kJ ⎞ ⎟⎟ (0°C − 100°C ) = (0.020 kg )⎜⎜ 2257 ⎟⎟ + (0.020 kg )⎜⎜ 4.184 kg K kg ⋅ ⎠ ⎝ ⎠ ⎝ = −45.14kJ − 8.37 kJ = −53.51 kJ Because Qsteam, max > Qmelt ice , all the ice will melt and the final temperature will be greater than 0°C. Apply conservation of energy to obtain: ∑Q i i or = Qcondense + Qcool the + Qmelt all + Qwarm the = 0 steam hot water the ice ice water − 45.14 kJ + mhot water c water (t f − 100°C ) + 50.03 kJ + mice c water (t f − 0°C ) = 0 Substituting numerical values yields: ⎛ kJ ⎞ ⎟ (t f − 100°C ) + 50.03 kJ − 45.14 kJ + (0.020 kg )⎜⎜ 4.18 kg ⋅ K ⎟⎠ ⎝ ⎛ kJ ⎞ ⎟ (t f − 0°C ) = 0 + (0.150 kg )⎜⎜ 4.184 kg ⋅ K ⎟⎠ ⎝ Solving for tf yields: t f = 4.9°C (b) Because the final temperature is greater than 0°C, no ice is left. 40 •• A calorimeter of negligible heat capacity contains 1.00 kg of water at 303 K and 50.0 g of ice at 273 K. (a) Find the final temperature T. (b) Find the final temperature T if the mass of ice is 500 g. 1796 Chapter 18 Picture the Problem First you need to convince yourself that there is enough energy in the water to melt all the ice. Once you’ve done that you can use conservation of energy to find the final equilibrium temperature. See Tables 18-1 and 18-2 for specific heats and the heat of fusion of water. (a) Find the heat available to melt the ice: ⎛ kJ ⎞ ⎟ (273 K − 303 K ) Qavailable, max = mwater c water ΔTwater = (1.00 kg )⎜⎜ 4.184 kg ⋅ K ⎟⎠ ⎝ = −125.5 kJ Find the heat required to melt all of the ice: ⎛ kJ ⎞ Qmelt ice = mice Lf = (0.0500 kg )⎜⎜ 333.5 ⎟⎟ = 16.68 kJ kg ⎠ ⎝ Because Qavailable, max > Qmelt ice , we know that the final temperature will be greater than 273 K. Apply conservation of energy to obtain: ∑Q i i = Qcool the warm water + Qmelt all + Qwarm the = 0 the ice ice water or (1.00 kg ) ⎛⎜⎜ 4.184 ⎝ kJ ⎞ ⎟ (T − 303 K ) + 16.68 kJ kg ⋅ K ⎟⎠ ⎛ kJ ⎞ ⎟ (T − 273 K ) = 0 + (0.0500 kg ) ⎜⎜ 4.184 kg ⋅ K ⎟⎠ ⎝ Solving for Tf yields: Tf = 298 K (b) Find the heat required to melt 500 g of ice: Qmelt ice = mice Lf = (0.500 kg )(333.5 kJ/kg ) ≈ 167 kJ Because the heat required to melt 500 g of ice is greater than the heat available, the final temperature will be 0°C. A 200-g aluminum calorimeter contains 600 g of water at 20.0ºC. A 41 •• 100-g piece of ice cooled to –20.0ºC is placed in the calorimeter. (a) Find the final temperature of the system, assuming no heat is transferred to or from the system. Heat and the First Law of Thermodynamics 1797 (b) A 200-g piece of ice at –20.0ºC is added. How much ice remains in the system after the system reaches equilibrium? (c) Would the answer for Part (b) change if both pieces of ice were added at the same time? Picture the Problem First you need to convince yourself that there is enough energy in the water and the calorimeter to melt all the ice. Once you’ve done that you can use conservation of energy to find the final equilibrium temperature in (a). In Part (b) you can find the energy required to raise the temperature of the 200 g of ice to 0°C and then, noting that there are now 700 g of water in the calorimeter, find the energy available from cooling the calorimeter and water from their equilibrium temperature to 0°C. Finally, you can find the amount of ice that will melt in terms of the difference between the energy available and the energy required to warm the ice. See Tables 18-1 and 18-2 for specific heats and the latent heat of fusion of water. Find the energy available to melt the ice: Qavailable, max = mwater cwater ΔTwater + mcal ccal ΔTwater = (mwater c water + mcal ccal )ΔTwater ⎡ ⎛ ⎛ kJ ⎞ kJ ⎞⎤ ⎟⎟+ (0.200 kg )⎜⎜ 0.900 ⎟⎥ (0°C − 20.0°C ) = ⎢(0.600 kg )⎜⎜ 4.184 kg ⋅ K ⎠ kg ⋅ K ⎟⎠⎦ ⎝ ⎝ ⎣ = 53.81 kJ Find the energy required to warm and melt all of the ice: Qwarm and = mice cice ΔTice + mice Lf melt the ice ⎛ ⎛ kJ ⎞ kJ ⎞ ⎟⎟ (0°C − (− 20.0°C )) + (0.100 kg )⎜⎜ 333.5 ⎟⎟ = (0.100 kg )⎜⎜ 2.05 kg ⋅ K ⎠ kg ⎠ ⎝ ⎝ = 37.45 kJ (a) Because Qavailable, max > Qmelt ice , we know that the final temperature will be greater than 0°C. Apply conservation of energy to obtain: ∑Q i i = Qcool the calorimeter + Qcool the warm water + Qwarm and + Qwarm the = 0 melt the ice ice water or, using the results from our preliminary calculations, mcalorimeter cCu (t f − 20.0°C ) + mwater c water (t f − 20.0°C ) + 37.45 kJ + mice c water (t f − 0°C ) = 0 1798 Chapter 18 Substituting numerical values yields: (0.200 kg )⎛⎜⎜ 0.900 ⎝ ⎛ kJ ⎞ kJ ⎞ ⎟⎟ (t f − 20.0°C ) + (0.600 kg )⎜⎜ 4.184 ⎟(t f − 20.0°C ) kg ⋅ K ⎠ kg ⋅ K ⎟⎠ ⎝ ⎛ kJ ⎞ ⎟ tf = 0 + 37.45 kJ + (0.100 kg )⎜⎜ 4.184 kg ⋅ K ⎟⎠ ⎝ Solve for tf to obtain: t f = 5.262°C = 5.26°C (b) The mass of ice remaining in the system when equilibrium is reached is the difference between the initial mass of ice and the mass of the ice that has melted: mice remaining = mice initially − mice melted = mice initially − Qavail − Qwarm ice to 0° C (1) Lf Find the energy required to raise the temperature of the 200 g of ice to 0°C: ⎛ kJ ⎞ ⎟ (0°C − (− 20.0°C )) = 8.080 kJ Qwarm ice = mice cice ΔTice = (0.200 kg )⎜⎜ 2.02 kg ⋅ K ⎟⎠ to 0° C ⎝ Noting that there are now 700 g of water in the calorimeter, find the energy available from cooling the calorimeter and water from 5.262°C to 0°C: Qavail = mwater cwater ΔTwater + mcal ccal ΔTwater = (mwater cwater + mcal ccal )ΔTwater ⎡ ⎛ ⎛ kJ ⎞ kJ ⎞⎤ ⎟⎟ + (0.200 kg )⎜⎜ 0.900 ⎟⎟⎥ (5.262°C − 0°C ) = ⎢(0.700 kg )⎜⎜ 4.184 ⋅ ⋅ kg K kg K ⎝ ⎠ ⎝ ⎠⎦ ⎣ = 16.359 kJ Substitute numerical values in equation (1) and evaluate mice : remaining mice remaining = 200 g − 16.359 kJ − 8.080 kJ = 200 g 333.5 kJ/kg (c) No. Because the initial and final conditions are the same, the answer would be the same. Heat and the First Law of Thermodynamics 1799 42 •• The specific heat of a 100-g block of a substance is to be determined. The block is placed in a 25-g copper calorimeter holding 60 g of water initially at 20ºC. Then, 120 mL of water at 80ºC are added to the calorimeter. When thermal equilibrium is reached, the temperature of the system is 54ºC. Determine the specific heat of the block. Picture the Problem Let the subscript B denote the block, w1 the water initially in the calorimeter, and w2 the 120 mL of water that is added to the calorimeter vessel. We can use conservation of energy to find the specific heat of the block. See Table 18-1 for specific heats. Apply conservation of energy to obtain: ∑Q i i = Qwarm the block + Qwarm the + Qwarm w1 + Qcool w 2 = 0 calorimeter or mB cB ΔT + mCu cCu ΔT + mw1 cw1 ΔT + mw 2 c w 2 ΔTw 2 = 0 where ΔT is the common temperature change of the calorimeter, block, and water initially in the calorimeter. Substitute numerical values to obtain: (0.100 kg )cB (54°C − 20°C) + (0.025 kg )⎛⎜⎜ 0.386 kJ ⎞ ⎟(54°C − 20°C ) kg ⋅ K ⎟⎠ ⎝ ⎛ kJ ⎞ ⎟(54°C − 20°C ) + (0.060 kg )⎜⎜ 4.184 kg ⋅ K ⎟⎠ ⎝ ⎛ kJ ⎞ ⎟ (54°C − 80°C ) = 0 + (0.120 kg )⎜⎜ 4.184 kg ⋅ K ⎟⎠ ⎝ Solving for cB yields: cB = 1.2 kJ/kg ⋅ K 43 •• [SSM] A 100-g piece of copper is heated in a furnace to a temperature tC. The copper is then inserted into a 150-g copper calorimeter containing 200 g of water. The initial temperature of the water and calorimeter is 16.0ºC, and the temperature after equilibrium is established is 38.0ºC. When the calorimeter and its contents are weighed, 1.20 g of water are found to have evaporated. What was the temperature tC? Picture the Problem We can find the temperature t by applying conservation of energy to this calorimetry problem. See Tables 18-1 and 18-2 for specific heats and the heat of vaporization of water. 1800 Chapter 18 Use conservation of energy to obtain: ∑Q i = Qvaporize + Qwarm water i the water + Qwarm the + Qcool the = 0 calorimeter Cu sample or mH 2 O, vaporized Lf, w + mH 2 O cH 2 O ΔTH 2 O + mcal ccal ΔTw + mCu cCu ΔTCu = 0 Substituting numerical values yields: ⎛ (1.20 g )⎜⎜ 2257 ⎝ ⎛ kJ ⎞ kJ ⎞ ⎟⎟ + (200 g )⎜⎜ 4.184 ⎟ (38.0°C − 16.0°C ) kg ⋅ K ⎠ kg ⋅ K ⎟⎠ ⎝ ⎛ ⎛ kJ ⎞ kJ ⎞ ⎟⎟ (38.0°C − 16.0°C ) + (100 g )⎜⎜ 0.386 ⎟ (38.0°C − t C ) = 0 + (150 g )⎜⎜ 0.386 kg ⋅ K ⎠ kg ⋅ K ⎟⎠ ⎝ ⎝ Solving for tC yields: t C = 618°C 44 •• A 200-g aluminum calorimeter contains 500 g of water at 20.0ºC. Aluminum shot with a mass equal to 300 g is heated to 100.0ºC and is then placed in the calorimeter. Find the final temperature of the system, assuming that there is no heat is transfer to the surroundings. Picture the Problem We can find the final temperature by applying conservation of energy to the calorimeter, the water in the calorimeter, and to the cooling aluminum shot. See Table 18-1 for the specific heats of aluminum and water. Use conservation of energy to obtain: ∑Q i i = Qwarm the + Qwarm the + Qcool the = 0 water calorimeter Al shot or mw c w ΔT + mcal cAl ΔT + mshot cAl ΔTAl = 0 where ΔT is the common temperature change of the water and calorimeter. Substituting numerical values yields: ⎡ ⎛ ⎛ kJ ⎞ kJ ⎞⎤ ⎟⎥ (t f − 20.0°C ) ⎟⎟+ (200 g )⎜⎜ 0.386 ⎢(500 g )⎜⎜ 4.184 kg ⋅ K ⎠ kg ⋅ K ⎟⎠⎦ ⎝ ⎝ ⎣ ⎛ kJ ⎞ ⎟ (t f − 100°C ) = 0 + (300 g )⎜⎜ 0.900 kg ⋅ K ⎟⎠ ⎝ Heat and the First Law of Thermodynamics Solve for tf to obtain: 1801 t f = 28.9°C First Law of Thermodynamics 45 • A diatomic gas does 300 J of work and also absorbs 2.50 kJ of heat. What is the change in internal energy of the gas? Picture the Problem We can apply the first law of thermodynamics to find the change in internal energy of the gas during this process. Apply the first law of thermodynamics to express the change in internal energy of the gas in terms of the heat added to the system and the work done on the gas: ΔEint = Qin + Won The work done by the gas equals the negative of the work done on the gas. Substitute numerical values and evaluate ΔEint: ΔEint = 2.50 kJ − 300 J = 2.20 kJ 46 • If a gas absorbs 1.67 MJ of heat while doing 800 kJ of work, what is the change in the internal energy of the gas? Picture the Problem We can apply the first law of thermodynamics to find the change in internal energy of the gas during this process. Apply the first law of thermodynamics to express the change in internal energy of the gas in terms of the heat added to the system and the work done on the gas: ΔEint = Qin + Won The work done by the gas is the negative of the work done on the gas. Substitute numerical values and evaluate ΔEint: ΔEint = 1.67 MJ − 800 kJ = 0.87 MJ 47 • If a gas absorbs 84 J while doing 30 J of work, what is the change in the internal energy of the gas? 1802 Chapter 18 Picture the Problem We can apply the first law of thermodynamics to find the change in internal energy of the gas during this process. Apply the first law of thermodynamics to express the change in internal energy of the gas in terms of the heat added to the system and the work done on the gas: ΔEint = Qin + Won The work done by the gas is the negative of the work done on the gas. Substitute numerical values and evaluate ΔEint: ΔEint = 84 J − 30 J = 54 J 48 •• A lead bullet initially at 30ºC just melts upon striking a target. Assuming that all of the initial kinetic energy of the bullet goes into the internal energy of the bullet, calculate the impact speed of the bullet. Picture the Problem We can use the definition of kinetic energy to express the speed of the bullet upon impact in terms of its kinetic energy. The heat absorbed by the bullet is the sum of the heat required to warm the bullet from 303 K to its melting temperature of 600 K and the heat required to melt it. We can use the first law of thermodynamics to relate the impact speed of the bullet to the change in its internal energy. See Table 18-1 for the specific heat and melting temperature of lead. Using the first law of thermodynamics, relate the change in the internal energy of the bullet to the work done on it by the target: Substitute for ΔEint, Kf, and Ki to obtain: Solving for v yields: ΔEint = Qin + Won or, because Qin = 0, ΔEint = Won = ΔK = −(K f − K i ) ( ) mcPb ΔTPb + mLf,Pb = − 0 − 12 mv 2 = 12 mv 2 or mcPb (TMP − Ti ) + mLf,Pb = 12 mv 2 v = 2[cPb (TMP − Ti ) + Lf,Pb ] Substitute numerical values and evaluate v: ⎧⎛ kJ ⎞ kJ ⎫ ⎟⎟ (600 K − 303 K ) + 24.7 ⎬ = 354 m/s v = 2⎨⎜⎜ 0.128 kg ⋅ K ⎠ kg ⎭ ⎩⎝ Heat and the First Law of Thermodynamics 1803 49 •• During a cold day, you can warm your hands by rubbing them together. Assume the coefficient of kinetic friction between your hands is 0.500, the normal force between your hands is 35.0 N, and that you rub them together at an average relative speed of 35.0 cm/s. (a) What is the rate at which mechanical energy is dissipated? (b) Assume further that the mass of each of your hands is 350 g, the specific heat of your hands is 4.00 kJ/kg⋅K, and that all the dissipated mechanical energy goes into increasing the temperature of your hands. How long must you rub your hands together to produce a 5.00°C increase in their temperature? Picture the Problem We can find the rate at which heat is generated when you rub your hands together using the definition of power and the rubbing time to produce a 5.00 °C increase in temperature from ΔQ = (dQ dt )Δt and Q = mcΔT. (a) The rate at which heat is generated as a function of the friction force and the average relative speed of your hands is given by: dQ = P = f k v = μFn v dt Substitute numerical values and evaluate dQ/dt: dQ = (0.500 )(35.0 N )(0.350 m/s ) dt = 6.13 W dQ mcΔT Δt = mcΔT ⇒ Δt = dQ dt dt (b) Relate the heat required to raise the temperature of your hands 5.00°C to the rate at which it is being generated: ΔQ = Substitute numerical values and evaluate Δt: ⎛ kJ ⎞ ⎟ (5.00 C°) 2(0.350 kg )⎜⎜ 4.00 kg ⋅ K ⎟⎠ ⎝ Δt = 6.13 W 1 min = 2286 s × = 38.1 min 60 s Work and the PV Diagram for a Gas 50 • The gas is allowed to expand at constant pressure until it reaches its final volume. It is then cooled at constant volume until it reaches its final pressure. (a) Illustrate this process on a PV diagram and calculate the work done by the gas. (b) Find the heat absorbed by the gas during this process. 1804 Chapter 18 Picture the Problem We can find the work done by the gas during this process from the area under the curve. Because no work is done along the constant volume (vertical) part of the path, the work done by the gas is done during its isobaric expansion. We can then use the first law of thermodynamics to find the heat added to the system during this process. (a) The path from the initial state (1) to the final state (2) is shown on the PV diagram. P, atm 1 3.00 2.00 2 1.00 0 0 1.00 2.00 3.00 V, L The work done by the gas equals the area under the shaded curve: 101.325 kPa ⎞ ⎛ 10 −3 m 3 ⎞ ⎛ ⎟ Wby gas = PΔV = (3.00 atm )(2.00 L ) = ⎜ 3.00 atm × ⎟ ⎜⎜ 2.00 L × atm L ⎟⎠ ⎝ ⎠⎝ = 608 J (b) The work done by the gas is the negative of the work done on the gas. Apply the first law of thermodynamics to the system to obtain: Substitute numerical values and evaluate Qin: Qin = ΔEint − Won = (Eint,2 − Eint,1 ) − (− Wby gas ) = (Eint,2 − Eint,1 ) + Wby gas Qin = (912 J − 456 J ) + 608 J = 1.06 kJ 51 • [SSM] The gas is first cooled at constant volume until it reaches its final pressure. It is then allowed to expand at constant pressure until it reaches its final volume. (a) Illustrate this process on a PV diagram and calculate the work done by the gas. (b) Find the heat absorbed by the gas during this process. Picture the Problem We can find the work done by the gas during this process from the area under the curve. Because no work is done along the constant volume (vertical) part of the path, the work done by the gas is done during its isobaric expansion. We can then use the first law of thermodynamics to find the heat absorbed by the gas during this process Heat and the First Law of Thermodynamics 1805 P, atm (a) The path from the initial state (1) to the final state (2) is shown on the PV diagram. 1 3.00 2.00 2 1.00 0 1.00 0 2.00 3.00 V, L The work done by the gas equals the area under the curve: ⎛ 101.325 kPa ⎞ 10 ⎛ Wby gas = PΔV = (2.00 atm )(2.00 L ) = ⎜ 2.00 atm × ⎟ ⎜⎜ 2.00 L × ⎝ atm ⎠⎝ m3 ⎞ ⎟ L ⎟⎠ −3 = 405 J (b) The work done by the gas is the negative of the work done on the gas. Apply the first law of thermodynamics to the system to obtain: Qin = ΔEint − Won Substitute numerical values and evaluate Qin: Q in = (912 J − 456 J ) + 405 J = 861 J = (Eint,2 − Eint,1 ) − (− Wby gas ) = (Eint,2 − Eint,1 ) + Wby gas 52 •• The gas is allowed to expand isothermally until it reaches its final volume and its pressure is 1.00 atm. It is then heated at constant volume until it reaches its final pressure. (a) Illustrate this process on a PV diagram and calculate the work done by the gas. (b) Find the heat absorbed by the gas during this process. Picture the Problem We can find the work done by the gas during this process from the area under the curve. Because no work is done along the constant volume (vertical) part of the path, the work done by the gas is done during its isothermal expansion. We can then use the first law of thermodynamics to find the heat absorbed by the gas during this process. 1806 Chapter 18 (a) The path from the initial state (1) to the final state (2) is shown on the PV diagram. P, atm 1 3.00 2.00 2 1.00 0 The work done by the gas equals the area under the curve: 0 1.00 V2 2.00 3.00 V, L 3L dV V 1L Wby gas = ∫ PdV = nRT1 ∫ V1 3.00 L = P1V1 dV 3.00 L = P1V1 [ln V ]1.00 L V 1.00 L ∫ = P1V1 ln 3 Substitute numerical values and evaluate Wby gas: 101.325 kPa ⎞ ⎛ 10 −3 m 3 ⎞ ⎛ ⎟ ln 3 = 334 J Wby gas = ⎜ 3.00 atm × ⎟ ⎜⎜1.00 L × atm L ⎟⎠ ⎝ ⎠⎝ (b) The work done by the gas is the negative of the work done on the gas. Apply the first law of thermodynamics to the system to obtain: Qin = ΔEint − Won Substitute numerical values and evaluate Qin: Q in = (912 J − 456 J ) + 334 J = 790 J = (Eint,2 − Eint,1 ) − (− Wby gas ) = (Eint,2 − Eint,1 ) + Wby gas 53 •• The gas is heated and is allowed to expand such that it follows a single straight-line path on a PV diagram from its initial state to its final state. (a) Illustrate this process on a PV diagram and calculate the work done by the gas. (b) Find the heat absorbed by the gas during this process. Picture the Problem We can find the work done by the gas during this process from the area under the curve. We can then use the first law of thermodynamics to find the heat absorbed by the gas during this process. Heat and the First Law of Thermodynamics (a) The path from the initial state (1) to the final state (2) is shown on the PV diagram: 1807 P, atm 1 3.00 2.00 2 1.00 0 0 The work done by the gas equals the area under the curve: 1.00 2.00 3.00 V,L Wby gas = Atrapezoid = 1 2 (3.00 atm + 2.00 atm)(2.00 L ) = 5.00 atm ⋅ L × 101.325 J atm ⋅ L = 507 J (b) The work done by the gas is the negative of the work done on the gas. Apply the first law of thermodynamics to the system to obtain: Qin = ΔEint − Won Substitute numerical values and evaluate Qin: Qin = (912 J − 456 J ) + 507 J = 963 J = (Eint,2 − Eint,1 ) − (− Wby gas ) = (Eint,2 − Eint,1 ) + Wby gas Remarks: You could use the linearity of the path connecting the initial and final states and the coordinates of the endpoints to express P as a function of V. You could then integrate this function between 1.00 and 3.00 L to find the work done by the gas as it goes from its initial to its final state. 54 •• In this problem, 1.00 mol of a dilute gas initially has a pressure equal to 1.00 atm and a volume equal to 25.0 L. As the gas is slowly heated, the plot of its state on a PV diagram moves in a straight line to the final state. The gas now has a pressure equal to 3.00 atm and a volume equal to 75.0 L. Find the work done and the heat absorbed by the gas. Picture the Problem We can find the work done by the gas during this process from the area under the curve and the heat absorbed by the gas from the 1st law of thermodynamics. 1808 Chapter 18 The path from the initial state i to the final state f is shown on the PV diagram: P, atm f 3.00 2.00 i 1.00 0 The work done by the gas equals the area under the curve: 0 25.0 50.0 75.0 V,L Wby gas = Atrapezoid = 1 2 (1.00 atm + 3.00 atm ) × (75.0 L − 25.0 L ) = 100 atm ⋅ L × 101.325 J atm ⋅ L = 10.1kJ The work done by the gas is the negative of the work done on the gas. Apply the first law of thermodynamics to the system to obtain: Qin = ΔEint − Won Substitute numerical values and evaluate Qin: Qin = (912 J − 456 J ) + 10.1 kJ = 10.6 kJ = (Eint,2 − Eint,1 ) − (− Wby gas ) = (Eint,2 − Eint,1 ) + Wby gas Remarks: You could use the linearity of the path connecting the initial and final states and the coordinates of the endpoints to express P as a function of V. You could then integrate this function between 25.0 and 75.0 L to find the work done by the gas as it goes from its initial to its final state. 55 •• In this problem, 1.00 mol of the ideal gas is heated while its volume changes, so that T = AP2, where A is a constant. The temperature changes from T0 to 4T0. Find the work done by the gas. Picture the Problem We can find the work done by the gas from the area under the PV curve provided we can find the pressure and volume coordinates of the initial and final states. We can find these coordinates by using the ideal gas law and the condition T = AP 2 . Heat and the First Law of Thermodynamics Apply the ideal-gas law with n = 1.00 mol and T = AP 2 to obtain: PV = RAP 2 ⇒ V = RAP (1) This result tells us that the volume varies linearly with the pressure. Solve T = AP2 for the pressure of the gas: P0 = T0 A Find the pressure when the temperature is 4T0: P= T 4T0 = 2 0 = 2 P0 A A Using equation (1), express the coordinates of the final state: The PV diagram for the process is shown to the right: 1809 (2V0 ,2 P0 ) P f 2P0 4T0 P0 i T0 V0 The work done by the gas equals the area under the curve: Wby gas = Atrapezoid = = 3 2 1 2 2V0 V (P0 + 2 P0 )(2V0 − V0 ) P0V0 56 •• A sealed, almost-empty spray paint can still contains a residual amount of the propellant: 0.020 mol of nitrogen gas. The can’s warning label clearly states: ″Do Not Dispose by Incineration.″ (a) Explain this warning and draw the PV diagram for the gas if, in fact, the can is subject to a high temperature. (b) You are in charge of testing the can. The manufacturer claims it can withstand an internal gas pressure of 6.00 atm before it breaks. The can is initially at roomtemperature and standard pressure in your testing laboratory. You begin to heat it uniformly using a heating element that has a power output of 200 W. The can and element are in an insulating oven, and you can assume 1.0% of the heat released by the heating element is absorbed by the gas in the can. How long should you expect the heating element to remain on before the can bursts? Picture the Problem This is a constant-volume process and so no work is done on the system. The heat that is added to the gas by the heating element increases the internal energy (and, hence, the temperature) of the gas. We can use the definition of power to relate the minimum time-to-bursting to the internal energy required to raise the temperature of the gas in the can and the rate at which energy is supplied by the heating element. The energy required to burst the can is related 1810 Chapter 18 to the increase in the temperature of the can through the definition of the heat capacity at constant volume Cv. P (a) Because the volume of the can is fixed (constant), the heat absorbed by the gas in the can all goes into increasing the internal energy of the gas and, thus, its temperature. At constant volume, the pressure rise is the maximum possible, threatening the structural integrity of the can and possibly leading to a grenade-like shrapnel explosion. Tf f Pf Ti Pi V ΔQ P (b) The heating time required to burst the container is related to the rate at which the heating element delivers energy to it: Δt = The heat that the heating element must supply to the gas is given by: ΔQ = C v ΔT = 52 nRΔT Substituting for ΔQ yields: i Δt = 5 2 nRΔT 5nRΔT = P 2P The temperature difference that corresponds to the bursting threshold is given by: ΔT = Tf − Ti Apply the ideal-gas law to the gas in the can to obtain: PiVi Pf Vf = Ti Tf or, because Vi = Vf, Pi Pf = Ti Tf Solving for the ratio of the final temperature to the initial temperature yields: Tf Pf = = 6 ⇒ Tf = 6Ti Ti Pi Substitute for Tf in equation (2) and evaluate ΔT: ΔT = 6Ti − Ti = 5Ti (1) (2) Heat and the First Law of Thermodynamics Substituting for ΔT in equation (1) yields: Δt = 1811 25nRTi 2P Substitute numerical values and evaluate Δt: J ⎞ ⎛ 25(0.020 mol)⎜ 8.314 ⎟ (293 K ) 1 min mol ⋅ K ⎠ ⎝ Δt = = 305 s ⋅ = 5.1 min 2(0.010)(200 W ) 60 s 57 •• [SSM] An ideal gas initially at 20ºC and 200 kPa has a volume of 4.00 L. It undergoes a quasi-static, isothermal expansion until its pressure is reduced to 100 kPa. Find (a) the work done by the gas, and (b) the heat absorbed by the gas during the expansion. Picture the Problem The PV diagram shows the isothermal expansion of the ideal gas from its initial state 1 to its final state 2. We can use the ideal-gas law for a fixed amount of gas to find V2 and then evaluate ∫ PdV for an isothermal process to find the work done by the gas. In Part (b) of the problem we can apply the first law of thermodynamics to find the heat added to the gas during the expansion. (a) Express the work done by a gas during an isothermal process: P, kPa 1 200 2 100 293 K 4.00 V2 V2 Apply the ideal-gas law for a fixed amount of gas undergoing an isothermal process: P1V1 = P2V2 ⇒ V2 = Substitute numerical values and evaluate V2: V2 = V 2 dV dV = P1V1 ∫ V V V1 V1 Wby gas = ∫ PdV = nRT ∫ V1 V, L V2 P1 V1 P2 200 kPa (4.00 L ) = 8.00 L 100 kPa 1812 Chapter 18 Substitute numerical values and evaluate W: 8.00 L dV 8.00 L = (800 kPa ⋅ L )[ln V ]4.00 L V 4.00 L Wby gas = (200 kPa )(4.00 L ) ∫ 10 −3 m 3 ⎛ 8.00 L ⎞ = (800 kPa ⋅ L ) ln⎜ ⎟ = 554.5 kPa ⋅ L × L ⎝ 4.00 L ⎠ = 555 J (b) Apply the first law of thermodynamics to the system to obtain: Because the work done by the gas is the negative of the work done on the gas: Qin = ΔEint − Won or, because ΔEint = 0 for an isothermal process, Qin = −Won Qin = −(− Wby gas ) = Wby gas = 555 J Remarks: in an isothermal expansion the heat added to the gas is always equal to the work done by the gas (ΔEint = 0). Heat Capacities of Gases and the Equipartition Theorem 58 • The heat capacity at constant volume of a certain amount of a monatomic gas is 49.8 J/K. (a) Find the number of moles of the gas. (b) What is the internal energy of the gas at T = 300 K? (c) What is the heat capacity at constant pressure of the gas? Picture the Problem We can find the number of moles of the gas from its heat capacity at constant volume using CV = 32 nR . We can find the internal energy of the gas from Eint = CVT and the heat capacity at constant pressure using CP = CV + nR . (a) Express CV in terms of the number of moles in the monatomic gas: C V = 32 nR ⇒ n = 2C V 3R Heat and the First Law of Thermodynamics Substitute numerical values and evaluate n: 1813 J⎞ ⎛ 2⎜ 49.8 ⎟ K⎠ ⎝ n= = 3.993 mol J ⎞ ⎛ 3⎜ 8.314 ⎟ mol ⋅ K ⎠ ⎝ = 3.99 mol (b) The internal energy of the gas is related to its temperature: Eint = C VT Substitute numerical values and evaluate Eint: J⎞ ⎛ Eint = ⎜ 49.8 ⎟ (300 K ) = 14.9 kJ K⎠ ⎝ (c) Relate the heat capacity at constant pressure to the heat capacity at constant volume: C P = C V + nR = 32 nR + nR = 52 nR Substitute numerical values and evaluate CP: CP = 5 2 (3.993 mol)⎛⎜ 8.314 ⎝ J ⎞ ⎟ mol ⋅ K ⎠ = 83.0 J/K 59 •• [SSM] The heat capacity at constant pressure of a certain amount of a diatomic gas is 14.4 J/K. (a) Find the number of moles of the gas. (b) What is the internal energy of the gas at T = 300 K? (c) What is the molar heat capacity of this gas at constant volume? (d) What is the heat capacity of this gas at constant volume? Picture the Problem (a) The number of moles of the gas is related to its heat capacity at constant pressure and its molar heat capacity at constant pressure according to C P = nc' P . For a diatomic gas, the molar heat capacity at constant pressure is c' P = 72 R . (b) The internal energy of a gas depends on its number of degrees of freedom and, for a diatomic gas, is given by Eint = 52 nRT . (c) The molar heat capacity of this gas at constant volume is related to its molar heat capacity at constant pressure according to c'V = c'P − R . (d) The heat capacity of this gas at constant volume is the product of the number of moles in the gas and its molar heat capacity at constant volume. (a) The number of moles of the gas is the ratio of its heat capacity at constant pressure to its molar heat capacity at constant pressure: n= CP c' P 1814 Chapter 18 J mol ⋅ K For a diatomic gas, the molar heat capacity is given by: c' P = 72 R = 29.1 Substitute numerical values and evaluate n: J K = 0.4948 mol n= J 29.1 mol ⋅ K 14.4 = 0.495 mol (b) With 5 degrees of freedom at this temperature: Eint = 52 nRT Substitute numerical values and evaluate Eint: Eint = 5 2 (0.4948 mol)⎛⎜ 8.314 ⎝ J ⎞ ⎟ (300 K ) = 3.09 kJ mol ⋅ K ⎠ (c) The molar heat capacity of this gas at constant volume is the difference between the molar heat capacity at constant pressure and the gas constant R: c'V = c'P − R Because c' P = 72 R for a diatomic gas: c' V = 72 R − R = 52 R Substitute the numerical value of R to obtain: J ⎞ J ⎛ c' V = 52 ⎜ 8.314 ⎟ = 20.79 mol ⋅ K ⎠ mol ⋅ K ⎝ J = 20.8 mol ⋅ K (d) The heat capacity of this gas at constant volume is given by: C' V = nc' V Substitute numerical values and evaluate C'V : J ⎞ ⎛ C' V = (0.4948 mol)⎜ 20.79 ⎟ mol ⋅ K ⎠ ⎝ J = 10.3 K 60 •• (a) Calculate the heat capacity per unit mass of air at constant volume and the heat capacity per unit mass of air at constant pressure. Assume that air has a temperature of 300 K and a pressure of 1.00 × 105 N/m2. Also assume that air is Heat and the First Law of Thermodynamics 1815 composed of 74.0% N2 molecules (molecular weight 28.0 g/mol) and 26.0% O2 molecules (molar mass of 32.0 g/mol) and that both components are ideal gases. (b) Compare your answer for the specific heat at constant pressure to the value listed in the Handbook of Chemistry and Physics of 1.032 kJ/kg⋅K. Picture the Problem The specific heats of air at constant volume and constant pressure are given by cV = CV/m and cP = CP/m and the heat capacities at constant volume and constant pressure are given by CV = 52 nR and CP = 72 nR , respectively. (a) Express the heat capacity per unit mass of air at constant volume and constant pressure: cV = CV m (1) CP m (2) and cP = CV = 52 nR Express the heat capacities of a diatomic gas in terms of the gas constant R, the number of moles n, and the number of degrees of freedom: and CP = 72 nR The mass of 1.00 mol of air is given by: m = 0.74 M N 2 + 0.26M O2 Substitute for CV and m in equation (1) to obtain: cV = 5nR 2 0.74M N2 + 0.26M O2 ( ) Substitute numerical values and evaluate cV: J ⎞ ⎛ 5(1.00 mol)⎜ 8.314 ⎟ J mol ⋅ K ⎠ ⎝ cV = = 716 −3 −3 2 (0.740) 28.0 × 10 kg + (0.260) 32.0 × 10 kg kg ⋅ K [ ( ) Substitute for CP and m in equation (2) to obtain: )] ( cP = 7nR 2 0.74 M N 2 + 0.26M O2 ( ) 1816 Chapter 18 Substitute numerical values and evaluate cP: J ⎞ ⎛ 7(1.00 mol)⎜ 8.314 ⎟ kJ mol ⋅ K ⎠ ⎝ cP = = 1.00 −3 −3 2 (0.740) 28.0 × 10 kg + (0.260) 32.0 × 10 kg kg ⋅ K [ ( ) ( )] (b) The percent difference between the value from the Handbook of Chemistry and Physics and the calculated value is: 1.032 kJ/kg ⋅ K − 1.00 kJ/kg ⋅ K ≈ 3% 1.032 J/kg ⋅ K In this problem, 1.00 mol of an ideal diatomic gas is heated at constant 61 •• volume from 300 to 600 K. (a) Find the increase in the internal energy of the gas , the work done by the gas, and the heat absorbed by the gas. (b) Find the same quantities if this gas is heated from 300 to 600 K at constant pressure. Use the first law of thermodynamics and your results for (a) to calculate the work done by the gas. (c) Again calculate the work done in Part (b). This time calculate it by integrating the equation dW = P dV. Picture the Problem (a) We know that, during a constant-volume process, no work is done and that we can calculate the heat added during this expansion from the heat capacity at constant volume and the change in the absolute temperature. We can then use the first law of thermodynamics to find the change in the internal energy of the gas. In Part (b), we can proceed similarly; using the heat capacity at constant pressure rather than constant volume. (a) The increase in the internal energy of a fixed amount of an ideal diatomic gas depends only on its change in temperature as it goes from one state to another: ΔEint = 52 nRΔT Substitute numerical values and evaluate ΔEint: ΔEint = 5 2 (1.00 mol)⎛⎜ 8.314 ⎝ For a constant-volume process: J ⎞ ⎟ (300 K ) = 6.236 kJ = 6.24 kJ mol ⋅ K ⎠ Won = 0 Heat and the First Law of Thermodynamics 1817 From the 1st law of thermodynamics we have: Qin = ΔEint − Won = 6.24 kJ − 0 (b) Because ΔEint depends only on the temperature difference: ΔEint = 6.24 kJ Relate the heat added to the gas to its heat capacity at constant pressure and the change in its temperature: = 6.24 kJ Qin = CP ΔT = ( 52 nR + nR )ΔT = 72 nRΔT Substitute numerical values and evaluate Qin: Qin = 7 2 (1.00 mol)⎛⎜ 8.314 ⎝ J ⎞ ⎟ (300 K ) = 8.730 kJ = 8.73 kJ mol ⋅ K ⎠ Apply the first law of thermodynamics to find W: (c) Integrate dWon = P dV to obtain: Won = ΔEint − Qin = 8.730 kJ − 6.236 kJ = 2.49 kJ Vf Won = ∫ PdV = P(Vf − Vi ) = nR(Tf − Ti ) Vi Substitute numerical values and evaluate Won: J ⎞ ⎛ Won = (1.00 mol)⎜ 8.314 ⎟ (300 K ) mol ⋅ K ⎠ ⎝ = 2.49 kJ 62 •• A diatomic gas is confined to a closed container of constant volume V0 and at a pressure P0. The gas is heated until its pressure triples. What amount of heat had to be absorbed by the gas in order to triple the pressure? Picture the Problem Because this is a constant-volume process, we can use Q = CV ΔT to express Q in terms of the temperature change and the ideal-gas law for a fixed amount of gas to find ΔT. Express the amount of heat Q that must be absorbed by the gas if its pressure is to triple: Q = CV ΔT = 52 nR(Tf − T0 ) 1818 Chapter 18 Using the ideal-gas law for a fixed amount of gas, relate the initial and final temperatures, pressures and volumes: P0V 3P0V = ⇒ Tf = 3T0 T0 Tf Substitute for Tf and simplify to obtain: Q = 52 nR(3T0 − T0 ) = 5(nRT0 ) = 5 P0V 63 •• In this problem, 1.00 mol of air is confined in a cylinder with a piston. The confined air is maintained at a constant pressure of 1.00 atm. The air is initially at 0ºC and has volume V0. Find the volume after 13,200 J of heat are absorbed by the trapped air. Picture the Problem Let the subscripts i and f refer to the initial and final states of the gas, respectively. We can use the ideal-gas law for a fixed amount of gas to express V′ in terms of V and the change in temperature of the gas when 13,200 J of heat are transferred to it. We can find this change in temperature using Q = C P ΔT . Using the ideal-gas law for a fixed amount of gas, relate the initial and final temperatures, volumes, and pressures: PiV Pf V' = Ti Tf ⎛ ΔT ⎞ Tf T + ΔT ⎟ =V i = V ⎜⎜1 + Ti Ti Ti ⎟⎠ ⎝ Noting that the process is isobaric, solve for V′ to obtain: V' = V Relate the heat transferred to the gas to the change in its temperature: Q = CP ΔT = 72 nRΔT ⇒ ΔT = Substitute for ΔT to obtain: ⎛ 2Q ⎞ ⎟⎟ V' = V ⎜⎜1 + ⎝ 7 nRTi ⎠ 2Q 7nR One mol of gas at standard temperature and pressure occupies 22.4 L. Substitute numerical values and evaluate V′: ⎛ ⎞ ⎜ ⎟ 2(13.2 kJ ) −3 3 ⎜ ⎟ = 59.6 L V' = 22.4 ×10 m 1 + ⎜ ⎟ J ⎞ ⎛ ⎟ (273 K ) ⎟ ⎜ 7(1.00 mol)⎜ 8.314 mol ⋅ K ⎠ ⎝ ⎝ ⎠ ( ) Heat and the First Law of Thermodynamics 1819 64 •• The heat capacity at constant pressure of a sample of gas is greater than the heat capacity at constant volume by 29.1 J/K. (a) How many moles of the gas are present? (b) If the gas is monatomic, what are Cv and Cp? (c) What are the values of Cv and Cp at normal room temperatures? Picture the Problem We can use the relationship between CP and CV ( C P = C V + nR ) to find the number of moles of this particular gas. In Parts (b) and (c) we can use the number of degrees of freedom associated with monatomic and diatomic gases, respectively, to find CP and CV. C P − CV R (a) Express the heat capacity of the gas at constant pressure to its heat capacity at constant volume: C P = C V + nR ⇒ n = Substitute numerical values and evaluate n: J K n= = 3.500 mol J 8.314 mol ⋅ K 29.1 = 3.50 mol (b) CV for a monatomic gas is given by: CV = 32 nR Substitute numerical values and evaluate CV: CV = 3 2 (3.500 mol)⎛⎜ 8.314 ⎝ J ⎞ ⎟ mol ⋅ K ⎠ = 43.65 J/K = 43.6 J/K Express CP for a monatomic gas: CP = 52 nR Substitute numerical values and evaluate CP: CP = 5 2 (3.500 mol)⎛⎜ 8.314 ⎝ = 72.7 J/K (c) At normal room temperature, if the diatomic molecules rotate but do not vibrate they have 5 degrees of freedom. Hence: C V = 52 nR and C P = C V + R = 72 nR J ⎞ ⎟ mol ⋅ K ⎠ 1820 Chapter 18 Substitute numerical values and evaluate CV and CP: CV = 5 2 (3.500 mol)⎛⎜ 8.314 ⎝ J ⎞ ⎟ mol ⋅ K ⎠ = 72.7 J/K and CP = 7 2 (3.500 mol)(8.314 J/mol ⋅ K ) = 102 J/K 65 •• [SSM] Carbon dioxide (CO2) at a pressure of 1.00 atm and a temperature of –78.5ºC sublimates directly from a solid to a gaseous state without going through a liquid phase. What is the change in the heat capacity at constant pressure per mole of CO2 when it undergoes sublimation? (Assume that the gas molecules can rotate but do not vibrate.) Is the change in the heat capacity positive or negative during sublimation? The CO2 molecule is pictured in Figure 18-22. Picture the Problem We can find the change in the heat capacity at constant pressure as CO2 undergoes sublimation from the energy per molecule of CO2 in the solid and gaseous states. Express the change in the heat capacity (at constant pressure) per mole as the CO2 undergoes sublimation: ΔCP = CP,gas − CP,solid Express Cp,gas in terms of the number of degrees of freedom per molecule: CP,gas = f ( 12 Nk ) = 52 Nk We know, from the Dulong-Petit Law, that the molar specific heat of most solids is 3R = 3Nk. This result is essentially a per-atom result as it was obtained for a monatomic solid with six degrees of freedom. Use this result and the fact CO2 is triatomic to express CP,solid: Substitute in equation (1) to obtain: (1) because each molecule has three translational and two rotational degrees of freedom in the gaseous state. CP,solid = 3Nk × 3 atoms = 9 Nk atom ΔC P = 52 Nk − 182 Nk = − 132 Nk 66 •• In this problem, 1.00 mol of a monatomic ideal gas is initially at 273 K and 1.00 atm. (a) What is the initial internal energy of the gas? (b) Find the work done by the gas when 500 J of heat are absorbed by the gas at constant pressure. Heat and the First Law of Thermodynamics 1821 What is the final internal energy of the gas? (c) Find the work done by the gas when 500 J of heat are absorbed by the gas at constant volume. What is the final internal energy of the gas? Picture the Problem We can find the initial internal energy of the gas from U i = 32 nRT and the final internal energy from the change in internal energy resulting from the addition of 500 J of heat. The work done during a constantvolume process is zero and the work done during the constant-pressure process can be found from the first law of thermodynamics. Eint,i = 32 nRT (a) Express the initial internal energy of the gas in terms of its temperature: Substitute numerical values and evaluate Eint,i: Eint,i = 3 2 (1.00 mol)⎛⎜ 8.314 ⎝ J ⎞ ⎟ (273 K ) = 3.405 kJ = 3.40 kJ mol ⋅ K ⎠ (b) Relate the final internal energy of the gas to its initial internal energy: Eint,f = Eint,i + ΔEint = Eint,i + CV ΔT Express the change in temperature of the gas resulting from the addition of heat: ΔT = Substitute to obtain: Eint,f = Eint,i + Substitute numerical values and evaluate Eint,f: ⎛ 3 nR ⎞ ⎟ (500 J ) Eint,f = 3.405 kJ + ⎜⎜ 25 ⎟ ⎝ 2 nR ⎠ Qin CP CV Qin CP = 3.705 kJ = 3.71 kJ The work done by the gas is the negative of the work done on the gas and is related to the change in internal energy and the heat added to the gas through the first law of thermodynamics: Wby gas = −Won = −ΔEint + Qin = −(Eint,f − Eint,i ) + Qin = − Eint,f + Eint,i + Qin 1822 Chapter 18 Substitute numerical values and evaluate Wby gas: Wby gas = −3.71 kJ + 3.40 kJ + 500 J (c) Relate the final internal energy of the gas to its initial internal energy: Eint,f = Eint,i + ΔEint Apply the first law of thermodynamics to the constantvolume process: ΔEint = Qin + Won Substitute numerical values and evaluate Eint,f: = 0.19 kJ or, because Won = 0, ΔEint = Qin = 500 J Eint,f = 3.405 kJ + 500 J = 3.91 kJ and, because Won = 0 = −Wby gas, Wby gas = 0 67 •• List all of the degrees of freedom possible for a water molecule and estimate the heat capacity of water at a temperature very far above its boiling point. (Ignore the fact the molecule might dissociate at high temperatures.) Think carefully about all of the different ways in which a water molecule can vibrate. Picture the Problem We can use CV,water = f ( 12 Nk ) to express CV,water and then count the number of degrees of freedom associated with a water molecule to determine f. Express CV,water in terms of the number of degrees of freedom per molecule: CV,water = f ( 12 Nk ) where f is the number of degrees of freedom associated with a water molecule. There are 3 translational degrees of freedom and three rotational degrees of freedom. In addition, each of the hydrogen atoms can vibrate against the oxygen atom, resulting in an additional 4 degrees of freedom (2 per atom). Substitute for f to obtain: C V,water = 10 ( 12 Nk ) = 5Nk = 5nR Heat Capacities of Solids and the Dulong-Petit Law 68 • The Dulong–Petit law was originally used to determine the molar mass of a substance from its measured heat capacity. The specific heat of a certain solid substance is measured to be 0.447 kJ/kg⋅K. (a) Find the molar mass of the substance. (b) What element has this specific heat value? Heat and the First Law of Thermodynamics 1823 Picture the Problem The Dulong-Petit law gives the molar specific heat of a solid, c′. The specific heat is defined as c = c′/M where M is the molar mass. Hence we can use this definition to find M and a periodic table to identify the element. 3R 3R ⇒M = c M (a) Apply the Dulong-Petit law: c' = 3R ⇒ c = Substitute numerical values and evaluate M: J mol ⋅ K = 55.7 g/mol M= kJ 0.447 kg ⋅ K 24.9 (b) Consulting the periodic table of elements we see that the element is most likely iron . Quasi-Static Adiabatic Expansion of a Gas 69 •• [SSM] A 0.500-mol sample of an ideal monatomic gas at 400 kPa and 300 K, expands quasi-statically until the pressure decreases to 160 kPa. Find the final temperature and volume of the gas, the work done by the gas, and the heat absorbed by the gas if the expansion is (a) isothermal and (b) adiabatic. Picture the Problem We can use the ideal-gas law to find the initial volume of the gas. In Part (a) we can apply the ideal-gas law for a fixed amount of gas to find the final volume and the expression for the work done in an isothermal process. Application of the first law of thermodynamics will allow us to find the heat absorbed by the gas during this process. In Part (b) we can use the relationship between the pressures and volumes for a quasi-static adiabatic process to find the final volume of the gas. We can apply the ideal-gas law to find the final temperature and, as in (a), apply the first law of thermodynamics, this time to find the work done by the gas. Use the ideal-gas law to express the initial volume of the gas: Vi = nRTi Pi Substitute numerical values and evaluate Vi: (0.500 mol)⎛⎜ 8.314 Vi = J ⎞ ⎟ (300 K ) mol ⋅ K ⎠ ⎝ = 3.118 × 10− 3 m 3 400 kPa 1824 Chapter 18 (a) Because the process is isothermal: Tf = Ti = 300 K Use the ideal-gas law for a fixed amount of gas to express Vf: PiVi Pf Vf = Ti Tf or, because T = constant, P Vf = Vi i Pf Substitute numerical values and evaluate Vf: ⎛ 400 kPa ⎞ ⎟⎟ = 7.795 L Vf = (3.118 L )⎜⎜ ⎝ 160 kPa ⎠ = 7.80 L Vf Vi Express the work done by the gas during the isothermal expansion: Wby gas = nRT ln Substitute numerical values and evaluate Wby gas: J ⎞ ⎛ Wby gas = (0.500 mol)⎜ 8.314 ⎟ mol ⋅ K ⎠ ⎝ ⎛ 7.795 L ⎞ ⎟⎟ × (300 K ) ln⎜⎜ ⎝ 3.118 L ⎠ = 1.14 kJ Noting that the work done by the gas during the process equals the negative of the work done on the gas, apply the first law of thermodynamics to find the heat absorbed by the gas: Qin = ΔEint − Won = 0 − (− 1.14 kJ ) = 1.14 kJ (b) Using γ = 5/3 and the relationship between the pressures and volumes for a quasi-static adiabatic process, express Vf: ⎛P PiVi = Pf Vf ⇒ Vf = Vi ⎜⎜ i ⎝ Pf Substitute numerical values and evaluate Vf: ⎛ 400 kPa ⎞ ⎟⎟ Vf = (3.118 L )⎜⎜ ⎝ 160 kPa ⎠ γ γ = 5.40 L 1γ ⎞ ⎟⎟ ⎠ 35 = 5.403 L Heat and the First Law of Thermodynamics Apply the ideal-gas law to find the final temperature of the gas: Tf = Pf Vf nR Substitute numerical values and evaluate Tf: Tf = (160 kPa )(5.403 ×10−3 m3 ) (0.500 mol)⎛⎜ 8.314 J ⎞⎟ ⎝ 1825 mol ⋅ K ⎠ = 208 K For an adiabatic process: Qin = 0 Apply the first law of thermodynamics to express the work done on the gas during the adiabatic process: Won = ΔEint − Qin = CV ΔT − 0 = 32 nRΔT Substitute numerical values and evaluate Won: Won = (0.500 mol)(8.314 J/mol ⋅ K ) × (208 K − 300 K ) 3 2 = −574 J Because the work done by the gas equals the negative of the work done on the gas: Wby gas = −(− 574 J ) = 574 J 70 •• A 0.500-mol sample of an ideal diatomic gas at 400 kPa and 300 K expands until the pressure decreases to 160 kPa. Find the final temperature and volume of the gas, the work done by the gas, and the heat absorbed by the gas if the expansion is (a) isothermal and (b) adiabatic. Picture the Problem We can use the ideal-gas law to find the initial volume of the gas. In Part (a) we can apply the ideal-gas law for a fixed amount of gas to find the final volume and the expression for the work done in an isothermal process. Application of the first law of thermodynamics will allow us to find the heat absorbed by the gas during this process. In Part (b) we can use the relationship between the pressures and volumes for a quasi-static adiabatic process to find the final volume of the gas. We can apply the ideal-gas law to find the final temperature and, as in (a), apply the first law of thermodynamics, this time to find the work done by the gas. Use the ideal-gas law to express the initial volume of the gas: Vi = nRTi Pi 1826 Chapter 18 Substitute numerical values and evaluate Vi: (0.500 mol)⎛⎜ 8.314 J ⎞ ⎟ (300 K ) mol ⋅ K ⎠ ⎝ 400 kPa Vi = = 3.118 L (a) Because the process is isothermal: Tf = Ti = 300 K Use the ideal-gas law for a fixed amount of gas to express Vf: PiVi Pf Vf = Ti Tf or, because T = constant, P Vf = Vi i Pf Substitute numerical values and evaluate Tf: ⎛ 400 kPa ⎞ ⎟⎟ = 7.795 L Vf = (3.118 L )⎜⎜ ⎝ 160 kPa ⎠ = 7.80 L Vf Vi Express the work done by the gas during the isothermal expansion: Wby gas = nRT ln Substitute numerical values and evaluate Wby gas: J ⎞ ⎛ Wby gas = (0.500 mol)⎜ 8.314 ⎟ mol ⋅ K ⎠ ⎝ ⎛ 7.795 L ⎞ ⎟⎟ × (300 K ) ln⎜⎜ ⎝ 3.118 L ⎠ = 1.14 kJ Noting that the work done by the gas during the isothermal expansion equals the negative of the work done on the gas, apply the first law of thermodynamics to find the heat absorbed by the gas: (b) Using γ = 1.4 and the relationship between the pressures and volumes for a quasi-static adiabatic process, express Vf: Qin = ΔEint − Won = 0 − (− 1.14 kJ ) = 1.14 kJ ⎛P PiVi = Pf Vf ⇒ Vf = Vi ⎜⎜ i ⎝ Pf γ γ 1γ ⎞ ⎟⎟ ⎠ Heat and the First Law of Thermodynamics Substitute numerical values and evaluate Vf: 1827 1 1.4 ⎛ 400 kPa ⎞ ⎟⎟ Vf = (3.118 L )⎜⎜ ⎝ 160 kPa ⎠ = 6.000 L = 6.00 L Apply the ideal-gas law to express the final temperature of the gas: Tf = Pf Vf nR Substitute numerical values and evaluate Tf: Tf = (160 kPa )(6.000 ×10−3 m3 ) (0.500 mol)(8.314 J/mol ⋅ K ) = 231 K For an adiabatic process: Qin = 0 Apply the first law of thermodynamics to express the work done on the gas during the adiabatic expansion: Won = ΔEint − Qin = CV ΔT − 0 = 52 nRΔT Substitute numerical values and evaluate Won: Won = (0.500 mol)(8.314 J/mol ⋅ K ) × (231 K − 300 K ) 5 2 = −717 J Noting that the work done by the gas during the adiabatic expansion is the negative of the work done on the gas, we have: Wby gas = −(− 717 J ) = 717 J 71 •• A 0.500-mol sample of helium gas expands adiabatically and quasistatically from an initial pressure of 5.00 atm and temperature of 500 K to a final pressure of 1.00 atm. Find (a) the final temperature of the gas, (b) the final volume of the gas, (c) the work done by the gas, and (d) the change in the internal energy of the gas. Picture the Problem We can eliminate the volumes from the equations relating the temperatures and volumes and the pressures and volumes for a quasi-static adiabatic process to obtain a relationship between the temperatures and pressures. We can find the initial volume of the gas using the ideal-gas law and the final volume using the pressure-volume relationship. In Parts (d) and (c) we can find the change in the internal energy of the gas from the change in its temperature and use the first law of thermodynamics to find the work done by the gas during its expansion. 1828 Chapter 18 (a) Express the relationship between temperatures and volumes for a quasi-static adiabatic process: TiVi γ −1 = Tf Vfγ −1 Express the relationship between pressures and volumes for a quasistatic adiabatic process: PiVi γ = Pf Vfγ Eliminate the volume between these two equations to obtain: ⎛P Tf = Ti ⎜⎜ f ⎝ Pi Substitute numerical values and evaluate Tf: ⎛ 1.00 atm ⎞ ⎟⎟ Tf = (500 K )⎜⎜ ⎝ 5.00 atm ⎠ (b) Solve equation (1) for Vf: ⎛P Vf = Vi ⎜⎜ i ⎝ Pf Apply the ideal-gas law to express Vi: Vi = (1) 1− γ1 ⎞ ⎟⎟ ⎠ 1− 513 = 263 K 1 ⎞γ ⎟⎟ ⎠ nRTi Pi Substitute numerical values and evaluate Vi: J ⎞ ⎟ (500 K ) mol ⋅ K ⎠ ⎝ Vi = 101.325 kPa 5.00atm × atm = 4.103 L Substitute for Vi and evaluate Vf: ⎛ 5.00 atm ⎞ 5 ⎟⎟ = 10.8 L Vf = (4.103 L )⎜⎜ ⎝ 1.00 atm ⎠ (c) The work done by the gas during an adiabatic expansion is the negative of the work done on the gas during the expansion: Because ΔEint = CV ΔT = 32 nRΔT : (0.500 mol)⎛⎜ 8.314 3 Wby the gas = −Won = −(ΔEint − Qin ) = −(ΔEint − 0) = −ΔEint Wby the gas = − 32 nRΔT Heat and the First Law of Thermodynamics 1829 Substitute numerical values and evaluate Wby the gas: J ⎞ ⎛ Wby the gas = − 32 (0.500 mol)⎜ 8.314 ⎟ (263 K − 500 K ) = 1.48 kJ mol ⋅ K ⎠ ⎝ (d) Apply the first law of thermodynamics for an adiabatic process to obtain: Substitute Wby the gas from (c): ΔEint = Qin + Won = 0 + (− Wby the gas ) = −Wby the gas ΔEint = −(1.48 kJ ) = − 1.48 kJ Cyclic Processes 72 •• A 1.00-mol sample of N2 gas at 20.0ºC and 5.00 atm is allowed to expand adiabatically and quasi-statically until its pressure equals 1.00 atm. It is then heated at constant pressure until its temperature is again 20.0ºC. After it reaches a temperature of 20.0ºC, it is heated at constant volume until its pressure is again 5.00 atm. It is then compressed at constant pressure until it is back to its original state. (a) Construct a PV diagram showing each process in the cycle. (b) From your graph, determine the work done by the gas during the complete cycle. (c) How much heat is absorbed (or released) by the gas during the complete cycle? Picture the Problem To construct the PV diagram we’ll need to determine the volume occupied by the gas at the beginning and ending points for each process. Let these points be A, B, C, and D. We can apply the ideal-gas law to the starting point (A) to find VA. To find the volume at point B, we can use the relationship between pressure and volume for a quasi-static adiabatic process. We can use the ideal-gas law to find the volume at point C and, because they are equal, the volume at point D. We can apply the first law of thermodynamics to find the amount of heat added to or subtracted from the gas during the complete cycle. (a) Using the ideal-gas law, express the volume of the gas at the starting point A of the cycle: Substitute numerical values and evaluate VA: VA = nRTA PA (1.00 mol)⎛⎜ 8.314 J ⎞ ⎟ (293 K ) mol ⋅ K ⎠ ⎝ VA = 101.325 kPa 5.00 atm × atm −3 3 = 4.808 × 10 m = 4.808 L 1830 Chapter 18 1 Use the relationship between pressure and volume for a quasistatic adiabatic process to express the volume of the gas at point B; the end point of the adiabatic expansion: ⎛ PA ⎞ γ VB = VA ⎜⎜ ⎟⎟ ⎝ PB ⎠ Substitute numerical values and evaluate VB: ⎛ 5.00 atm ⎞ 1.4 ⎟⎟ = 15.18 L VB = (4.808 L )⎜⎜ ⎝ 1.00 atm ⎠ Using the ideal-gas law for a fixed amount of gas, express the volume occupied by the gas at points C and D: VC = VD = Substitute numerical values and evaluate VC: The complete cycle is shown in the diagram. 1 nRTC PC (1.00 mol)⎛⎜ 8.314 J ⎞ ⎟ (293 K ) mol ⋅ K ⎠ ⎝ VC = 101.325 kPa 1.00 atm × atm −3 3 = 24.04 × 10 m = 24.04 L P , atm A 5.00 0 4.00 3.00 2.00 1.00 0 B 0 5 10 15 C 20 25 V,L (b) Note that for the paths A→B and B→C, Wby gas, the work done by the gas, is positive. For the path D→A, Wby gas is negative, and greater in magnitude than WA→C. Therefore the total work done by the gas is negative. Find the area enclosed by the cycle by noting that each rectangle of dotted lines equals 5 atm⋅L and counting the rectangles: ⎛ atm ⋅ L ⎞ ⎛ 101.325 J ⎞ ⎟⎟ = (− 65 atm ⋅ L )⎜ Wby gas ≈ −(13 rectangles)⎜⎜ 5.00 ⎟ = −6.586 J rectangle ⎠ ⎝ atm ⋅ L ⎠ ⎝ = − 6.6 kJ Heat and the First Law of Thermodynamics (c) The work done on the gas equals the negative of the work done by the gas. Apply the first law of thermodynamics to find the amount of heat added to or subtracted from the gas during the complete cycle: 1831 Qin = ΔEint − Won = 0 − (− 6.59 kJ ) = 6.6 kJ because ΔEint = 0 for the complete cycle. 73 •• [SSM] A 1.00-mol sample of an ideal diatomic gas is allowed to expand. This expansion is represented by the straight line from 1 to 2 in the PV diagram (Figure 18-23). The gas is then compressed isothermally. This compression is represented by the straight line from 2 to 1 in the PV diagram. Calculate the work per cycle done by the gas. Picture the Problem The total work done as the gas is taken through this cycle is the area bounded by the two processes. Because the process from 1→2 is linear, we can use the formula for the area of a trapezoid to find the work done during this expansion. We can use Wisothermal process = nRT ln (Vf Vi ) to find the work done on the gas during the process 2→1. The net work done during this cycle is then the sum of these two terms. Express the net work done per cycle: Wnet = Wby the gas + Won the gas = W1→ 2 + W2→1 Work is done by the gas during its expansion from 1 to 2 and hence is equal to the negative of the area of the trapezoid defined by this path and the vertical lines at V1 = 11.5 L and V2 = 23 L. Use the formula for the area of a trapezoid to express W1→2: W1→ 2 = − Atrap Work is done on the gas during the isothermal compression from V2 to V1 and hence is equal to the area under the curve representing this process. Use the expression for the work done during an isothermal process to express W2→1: ⎛V W2→1 = nRT ln⎜⎜ f ⎝ Vi = − 12 (23 L − 11.5 L ) (1) × (2.0 atm + 1.0 atm ) = −17.3 L ⋅ atm ⎞ ⎟⎟ ⎠ 1832 Chapter 18 Apply the ideal-gas law at point 1 to find the temperature along the isotherm 2→1: T= (2.0 atm )(11.5 L ) PV = = 280 K nR (1.00 mol) 8.206 ×10 − 2 L ⋅ atm/mol ⋅ K ( ) Substitute numerical values and evaluate W2→1: ⎛ 11.5 L ⎞ ⎟⎟ = 15.9 L ⋅ atm W2→1 = (1.00 mol) 8.206 × 10 −2 L ⋅ atm/mol ⋅ K (280 K )ln⎜⎜ ⎝ 23 L ⎠ ( Substitute in equation (1) and evaluate Wnet: ) Wnet = −17.3 L ⋅ atm + 15.9 L ⋅ atm = −1.40 L ⋅ atm × 101.325 J L ⋅ atm = − 0.14 kJ 74 •• A 2.00-mol sample of an ideal monatomic gas have an initial pressure of 2.00 atm and an initial volume of 2.00 L. The gas is taken through the following quasi-static cycle: It is expanded isothermally until it has a volume of 4.00 L. It is next heated at constant volume until it has a pressure of 2.00 atm. It is then cooled at constant pressure until it is back to its initial state. (a) Show this cycle on a PV diagram. (b) Find the temperatures at each end of each part of the cycle. (c) Calculate the heat absorbed and the work done by the gas during each part of the cycle. Picture the Problem Denote the initial and final points of each part of the cycle by the numerals 1, 2 and 3. We can apply the ideal-gas law to find the temperatures T1, T2, and T3. We can use the appropriate work and heat equations to calculate the heat added and the work done by the gas for the isothermal process (1→2), the constant-volume process (2→3), and the isobaric process (3→1). (a) The cycle is shown in the PV diagram to the right: P , atm 1 2.00 3 1.00 0 2 0 1.00 2.00 3.00 4.00 V,L Heat and the First Law of Thermodynamics (b) Use the ideal-gas law to find T1: T1 = = P1V1 nR 1833 (2.00 atm)(2.00 L ) (2.00 mol)⎛⎜ 8.206 ×10 − 2 ⎝ L ⋅ atm ⎞ ⎟ mol ⋅ K ⎠ = 24.37 K = 24.4 K Because the process 1→2 is isothermal: T2 = 24.4 K Use the ideal-gas law to find T3: T3 = = P3V3 nR (2.00 atm)(4.00 L ) (2.00 mol)⎛⎜ 8.206 ×10 − 2 ⎝ L ⋅ atm ⎞ ⎟ mol ⋅ K ⎠ = 48.74 K = 48.7 K (c) Because the process 1→2 is isothermal, Qin,1→2 = Wby gas,1→2: ⎛V ⎞ Qin, 1→2 = Wby gas,1→2 = nRT ln⎜⎜ 2 ⎟⎟ ⎝ V1 ⎠ Substitute numerical values and evaluate Qin,1→2: ⎛ 4.00 L ⎞ J ⎞ ⎛ ⎟⎟ = 281 J Qin, 1→2 = (2.00 mol)⎜ 8.314 ⎟ (24.37 K ) ln⎜⎜ mol ⋅ K ⎠ 2.00 L ⎝ ⎝ ⎠ Because process 2→3 takes place at constant volume: W2→3 = 0 Because process 2→3 takes place at constant volume, Won,2→3 = 0, and: Qin,2→3 = ΔEint,2→3 = C V ΔT = 32 nR(T3 − T2 ) Substitute numerical values and evaluate Qin,2→3: Qin,2→3 = 3 2 (2.00 mol)⎛⎜ 8.314 ⎝ Because process 3→1 is isobaric: J ⎞ ⎟ (48.74 K − 24.37 K ) = 608 J mol ⋅ K ⎠ Q3→1 = C P ΔT = 52 nR(T1 − T3 ) 1834 Chapter 18 Substitute numerical values and evaluate Q3→1: Q3→1 = 5 2 (2.00 mol)⎛⎜ 8.314 ⎝ J ⎞ ⎟ (24.37 K − 48.74 K ) = − 1.01 kJ mol ⋅ K ⎠ The work done by the gas from 3 to 1 equals the negative of the work done on the gas: Wby gas,3→1 = − P1,3 ΔV = P1,3 (V1 − V3 ) Substitute numerical values and evaluate Wby gas,3→2: ⎛ 101.325 J ⎞ Wby gas,3→1 = −(2.00 atm )(2.00 L − 4.00 L ) = −(− 4.00 atm ⋅ L )⎜ ⎟ ⎝ atm ⋅ L ⎠ = 405 J 75 ••• [SSM] At point D in Figure 18-24 the pressure and temperature of 2.00 mol of an ideal monatomic gas are 2.00 atm and 360 K, respectively. The volume of the gas at point B on the PV diagram is three times that at point D and its pressure is twice that at point C. Paths AB and CD represent isothermal processes. The gas is carried through a complete cycle along the path DABCD. Determine the total amount of work done by the gas and the heat absorbed by the gas along each portion of the cycle. Picture the Problem We can find the temperatures, pressures, and volumes at all points for this ideal monatomic gas (3 degrees of freedom) using the ideal-gas law and the work for each process by finding the areas under each curve. We can find the heat exchanged for each process from the heat capacities and the initial and final temperatures for each process. Express the total work done by the gas per cycle: Wby gas,tot = WD→A + WA→B + WB→C + WC→D 1. Use the ideal-gas law to find the volume of the gas at point D: VD = nRTD PD (2.00 mol)⎛⎜ 8.314 J ⎞ ⎟ (360 K ) mol ⋅ K ⎠ ⎝ = (2.00 atm )⎛⎜101.325 kPa ⎞⎟ atm ⎠ ⎝ = 29.54 L Heat and the First Law of Thermodynamics 1835 VB = VC = 3VD = 3(29.54 L ) 2. We’re given that the volume of the gas at point B is three times that at point D: = 88.62 L Use the ideal-gas law to find the pressure of the gas at point C: PC = nRTC = VC (2.00 mol)⎛⎜ 8.206 ×10 − 2 ⎝ L ⋅ atm ⎞ ⎟ (360 K ) mol ⋅ K ⎠ 88.62 L We’re given that the pressure at point B is twice that at point C: = 0.6667 atm PB = 2 PC = 2(0.6667 atm ) = 1.333 atm 3. Because path DC represents an isothermal process: TD = TC = 360 K Use the ideal-gas law to find the temperatures at points B and A: TA = TB = Because the temperature at point A is twice that at D and the volumes are the same, we can conclude that: PA = 2 PD = 4.00 atm The pressure, volume, and temperature at points A, B, C, and D are summarized in the table to the right. 4. For the path D→A, WD→A = 0 and: PBVB nR (1.333 atm)(88.62 L ) = (2.00 mol)⎛⎜ 8.206 ×10 − 2 L ⋅ atm ⎞⎟ mol ⋅ K ⎠ ⎝ = 719.8 K Point A B C D P (atm) 4.00 1.33 0.667 2.00 V (L) 29.5 88.6 88.6 29.5 T (K) 720 720 360 360 QD→A = ΔEint, D→A = 32 nRΔTD→A = 32 nR(TA − TD ) 1836 Chapter 18 Substitute numerical values and evaluate QD→A: QD→A = 3 2 (2.00 mol)⎛⎜ 8.314 ⎝ For the path A→B: J ⎞ ⎟ (720 K − 360 K ) = 8.979 kJ mol ⋅ K ⎠ ⎛V WA→B = QA→B = nRTA,B ln⎜⎜ B ⎝ VA ⎞ ⎟⎟ ⎠ Substitute numerical values and evaluate WA→B: ⎛ 88.62 L ⎞ J ⎞ ⎛ ⎟⎟ = 13.15 kJ WA→B = (2.00 mol)⎜ 8.314 ⎟ (720 K )ln⎜⎜ mol ⋅ K ⎠ ⎝ ⎝ 29.54 L ⎠ and, because process A→B is isothermal, ΔEint, A→B = 0 For the path B→C, WB→C = 0 , and: QB→C = ΔU B→C = C V ΔT = 32 nR(TC − TB ) Substitute numerical values and evaluate QB→C: QB→C = 3 2 (2.00 mol)(8.314 J/mol ⋅ K )(360 K − 720 K ) = −8.979 kJ For the path C→D: ⎛V ⎞ WC→D = nRTC,D ln⎜⎜ D ⎟⎟ ⎝ VC ⎠ Substitute numerical values and evaluate WC→D: ⎛ 29.54 L ⎞ J ⎞ ⎛ ⎟⎟ = −6.576 kJ WC→D = (2.00 mol)⎜ 8.314 ⎟ (360 K ) ln⎜⎜ mol ⋅ K ⎠ ⎝ ⎝ 88.62 L ⎠ Also, because process A→B is isothermal, ΔEint,A→B = 0 , and QC→D = WC→D = −6.58 kJ Qin, Won, and ΔEint are summarized for each of the processes in the table to the right. 8.98 Won (kJ) 0 ΔEint (kJ) 8.98 A→B 13.2 −13.2 0 B→C − 8.98 0 −8.98 Process Qin (kJ) D→A Heat and the First Law of Thermodynamics C→D − 6.58 6.58 1837 0 Referring to the table, find the total work done by the gas per cycle: Wby gas, tot = WD→ A + WA →B + WB→C + WC →D = 0 + 13.2 kJ + 0 − 6.58 kJ = 6.6 kJ Remarks: Note that, as it should be, ΔEint is zero for the complete cycle. 76 ••• At point D in Figure 18-24 the pressure and temperature of 2.00 mol of an ideal diatomic gas are 2.00 atm and 360 K, respectively. The volume of the gas at point B on the PV diagram is three times that at point D and its pressure is twice that at point C. Paths AB and CD represent isothermal processes. The gas is carried through a complete cycle along the path DABCD. Determine the total amount of work done by the gas and the heat absorbed by the gas along each portion of the cycle. Picture the Problem We can find the temperatures, pressures, and volumes at all points for this ideal diatomic gas (5 degrees of freedom) using the ideal-gas law and the work for each process by finding the areas under each curve. We can find the heat exchanged for each process from the heat capacities and the initial and final temperatures for each process. Express the total work done by the gas per cycle: Wby gas,tot = WD→A + WA→B + WB→C + WC→D 1. Use the ideal-gas law to find the volume of the gas at point D: VD = nRTD PD (2.00 mol)⎛⎜ 8.314 J ⎞ ⎟ (360 K ) mol ⋅ K ⎠ ⎝ = (2.00 atm )⎛⎜101.325 kPa ⎞⎟ atm ⎠ ⎝ = 29.54 L 2. We’re given that the volume of the gas at point B is three times that at point D: VB = VC = 3VD = 3(29.54 L ) = 88.62 L 1838 Chapter 18 Use the ideal-gas law to find the pressure of the gas at point C: PC = nRTC = VC (2.00 mol)⎛⎜ 8.206 ×10 − 2 ⎝ L ⋅ atm ⎞ ⎟(360 K ) mol ⋅ K ⎠ 88.62 L = 0.6667 atm PB = 2 PC = 2(0.6667 atm ) = 1.333 atm We’re given that the pressure at point B is twice that at point C: 3. Because path DC represents an isothermal process: TD = TC = 360 K Use the ideal-gas law to find the temperatures at points B and A: TA = TB = Because the temperature at point A is twice that at D and the volumes are the same, we can conclude that: PA = 2 PD = 4.00 atm PBVB nR (1.333 atm)(88.62 L ) = (2.00 mol)⎛⎜ 8.206 ×10 − 2 L ⋅ atm ⎞⎟ mol ⋅ K ⎠ ⎝ = 719.8 K The pressure, volume, and temperature at points A, B, C, and D are summarized in the table to the right. Point A B C D P (atm) 4.00 1.33 0.667 2.00 V (L) 29.5 88.6 88.6 29.5 4. For the path D→A, WD→A = 0 and: QD→A = ΔU D→A = 52 nRΔTD→A = 52 nR(TA − TD ) = 5 2 (2.00 mol)⎛⎜ 8.314 = 14.97 kJ ⎝ J ⎞ ⎟ (720 K − 360 K ) mol ⋅ K ⎠ T (K) 720 720 360 360 Heat and the First Law of Thermodynamics 1839 For the path A→B: ⎛V ⎞ ⎛ 88.62 L ⎞ J ⎞ ⎛ ⎟⎟ WA → B = QA → B = nRTA,B ln⎜⎜ B ⎟⎟ = (2.00 mol)⎜ 8.314 ⎟(720 K )ln⎜⎜ mol K V 29.54 L ⋅ ⎠ ⎝ ⎠ ⎝ ⎝ A⎠ = 13.15 kJ and, because process A→B is isothermal, ΔEint,A→B = 0 For the path B→C, WB→C = 0 and: QB→C = ΔU B→C = C V ΔT = 52 nR(TC − TB ) = 5 2 (2.00 mol)⎛⎜ 8.314 ⎝ J ⎞ ⎟ (360 K − 720 K ) mol ⋅ K ⎠ = −14.97 kJ For the path C→D: ⎛V ⎞ ⎛ 29.54 L ⎞ J ⎞ ⎛ ⎟⎟ = −6.576 kJ WC→D = nRTC,D ln⎜⎜ D ⎟⎟ = (2.00 mol)⎜ 8.314 ⎟ (360 K )ln⎜⎜ mol ⋅ K ⎠ ⎝ ⎝ 88.62 L ⎠ ⎝ VC ⎠ Also, because process A→B is isothermal, ΔEint,A→B = 0 and QC→D = WC→D = −6.576 kJ Qin, Won, and ΔEint are summarized for each of the processes in the table to the right. 15.0 Won (kJ) 0 ΔEint (kJ) 15.0 A→B 13.2 −13.2 0 B→C − 15.0 0 −15.0 C→D − 6.58 6.58 0 Process Qin (kJ) D→A Referring to the table and noting that the work done by the gas equals the negative of the work done on the gas, find the total work done by the gas per cycle: Wby gas,tot = WD→A + WA→B + WB→C + WC→D = 0 + 13.2 kJ + 0 − 6.58 kJ = 6.6 kJ 1840 Chapter 18 Remarks: Note that ΔEint for the complete cycle is zero and that the total work done is the same for the diatomic gas of this problem and the monatomic gas of problem 89. 77 ••• A sample consisting of n moles of an ideal gas is initially at pressure P1, volume V1, and temperature Th. It expands isothermally until its pressure and volume are P2 and V2. It then expands adiabatically until its temperature is Tc and its pressure and volume are P3 and V3. It is then compressed isothermally until it is at a pressure P4 and a volume V4, which is related to its initial volume V1 by TcV4γ −1 = ThV1γ −1 . The gas is then compressed adiabatically until it is back in its original state. (a) Assuming that each process is quasi-static, plot this cycle on a PV diagram. (This cycle is known as the Carnot cycle for an ideal gas.) (b) Show that the heat Qh absorbed during the isothermal expansion at Th is Qh = nRTh ln(V2/V1). (c) Show that the heat Qc released by the gas during the isothermal compression at Tc is Qc = nRTc ln (V3/V4). (d) Using the result that TVγ–1 is constant for a quasi-static adiabatic expansion, show that V2/V1 = V3/V4. (e) The efficiency of a Carnot cycle is defined as the net work done by the gas, divided by the heat absorbed Qh by the gas. Using the first law of thermodynamics, show that the efficiency is 1 – Qc/Qh. (f) Using your results from the previous parts of this problem, show that Qc/Qh = Tc/Th. Picture the Problem We can use the equations of state for adiabatic and isothermal processes to express the work done on or by the system, the heat entering or leaving the system, and the change in internal energy for each of the four processes making up the Carnot cycle. We can use the first law of thermodynamics and the definition of the efficiency of a Carnot cycle to show that the efficiency is 1 – Qc / Qh. (a) The cycle is shown on the PV diagram to the right: P P1 1 2 P2 Th 4 P4 Tc P3 3 V1 (b) Because the process 1→2 is isothermal: ΔEint, 1→2 = 0 V4 V2 V3 V Heat and the First Law of Thermodynamics Apply the first law of thermodynamics to obtain: ⎛V ⎞ Qh = Q1→2 = W1→2 = nRTh ln⎜⎜ 2 ⎟⎟ ⎝ V1 ⎠ (c) Because the process 3→4 is isothermal: ΔU 3→4 = 0 Apply the first law of thermodynamics to obtain: ⎛V ⎞ Qc = Q3→4 = W3→4 = nRTc ln⎜⎜ 4 ⎟⎟ ⎝ V3 ⎠ 1841 ⎛V ⎞ = − nRTc ln⎜⎜ 3 ⎟⎟ ⎝ V4 ⎠ where the minus sign tells us that heat is given off by the gas during this process. (d) Apply the equation for a quasi-static adiabatic process at points 4 and 1 to obtain: 1 γ −1 γ −1 = ThV1 TcV4 γ −1 V ⎛T ⎞ ⇒ 1 = ⎜⎜ c ⎟⎟ (1) V4 ⎝ Th ⎠ 1 Apply the equation for a quasistatic adiabatic process at points 2 and 3 to obtain: ThV2 Equate equations (1) and (2) and rearrange to obtain: V3 V = 2 V4 V1 (e) Express the efficiency of the Carnot cycle: ε= Apply the first law of thermodynamics to obtain: Won = ΔEint, cycle − Qin Substitute to obtain: γ −1 γ −1 = TcV3 γ −1 V2 ⎛ Tc ⎞ ⇒ = ⎜⎜ ⎟⎟ (2) V3 ⎝ Th ⎠ W Qh = 0 − (Qh − Qc ) = −(Qh − Qc ) because Eint is a state function and ΔEint, cycle = 0 . ε= Wby the gas Qh = 1− Qc Qh = − Won Qh − Qc = Qh Qh 1842 Chapter 18 (f) In Part (b) we established that: ⎛V ⎞ Qh = nRTh ln⎜⎜ 2 ⎟⎟ ⎝ V1 ⎠ In Part (c) we established that the heat leaving the system along the path 3→4 is given by: ⎛V ⎞ Qc = nRTc ln⎜⎜ 3 ⎟⎟ ⎝ V4 ⎠ Divide the second of these equations by the first to obtain: ⎛V ⎞ nRTc ln⎜⎜ 3 ⎟⎟ Qc ⎝ V4 ⎠ = Tc = Th Qh ⎛V ⎞ nRTh ln⎜⎜ 2 ⎟⎟ ⎝ V1 ⎠ because V3 V2 = . V4 V1 Remarks: This last result establishes that the efficiency of a Carnot cycle is T also given by εC = 1 − c . Th General Problems 78 • During the process of quasi-statically compressing an ideal diatomic gas to one-fifth of its initial volume, 180 kJ of work are done on the gas. (a) If this compression is accomplished isothermally at room temperature (293 K), how much heat released by the gas? (b) How many moles of gas are in this sample? Picture the Problem (a) We can use the first law of thermodynamics to relate the heat removed from the gas to the work done on the gas. (b) We can find the number of moles of the gas from the expression for the work done on or by a gas during an isothermal process. (a) Apply the first law of thermodynamics to this process: Qin = ΔEint − Won = −Won because ΔEint = 0 for an isothermal process. Substitute numerical values to obtain: Qin = −180 kJ Because Qremoved = −Qin: Qremoved = 180 kJ Heat and the First Law of Thermodynamics (b) The work done on the gas during the isothermal process is given by: ⎛V W = nRT ln⎜⎜ f ⎝ Vi Substitute numerical values and evaluate n: n= ⎞ W ⎟⎟ ⇒ n = ⎛V ⎠ RT ln⎜⎜ f ⎝ Vi 1843 ⎞ ⎟⎟ ⎠ − 180 kJ J ⎞ ⎛1⎞ ⎛ ⎟ (293 K )ln⎜ ⎟ ⎜ 8.314 mol ⋅ K ⎠ ⎝5⎠ ⎝ = 45.9 mol 79 • [SSM] The PV diagram in Figure 18-25 represents 3.00 mol of an ideal monatomic gas. The gas is initially at point A. The paths AD and BC represent isothermal changes. If the system is brought to point C along the path AEC, find (a) the initial and final temperatures of the gas, (b) the work done by the gas, and (c) the heat absorbed by the gas. Picture the Problem We can use the ideal-gas law to find the temperatures TA and TC. Because the process EDC is isobaric, we can find the area under this line geometrically and then use the 1st law of thermodynamics to find QAEC. (a) Using the ideal-gas law, find the temperature at point A: TA = = PAVA nR (4.0 atm )(4.01 L ) (3.00 mol)⎛⎜ 8.206 ×10 − 2 ⎝ L ⋅ atm ⎞ ⎟ mol ⋅ K ⎠ = 65.2 K = 65 K Using the ideal-gas law, find the temperature at point C: TC = = PCVC nR (1.0 atm )(20.0 L ) (3.00 mol)⎛⎜ 8.206 ×10 − 2 ⎝ L ⋅ atm ⎞ ⎟ mol ⋅ K ⎠ = 81.2 K = 81 K (b) Express the work done by the gas along the path AEC: WAEC = WAE + WEC = 0 + PEC ΔVEC = (1.0 atm )(20.0 L − 4.01 L ) 101.325 J = 15.99 L ⋅ atm × L ⋅ atm = 1.62 kJ = 1.6 kJ 1844 Chapter 18 (c) Apply the first law of thermodynamics to express QAEC: QAEC = WAEC + ΔEint = WAEC + CV ΔT = WAEC + 32 nRΔT = WAEC + 32 nR(TC − TA ) Substitute numerical values and evaluate QAEC: QAEC = 1.62 kJ + 32 (3.00 mol)(8.314 J/mol ⋅ K )(81.2 K − 65.2 K ) = 2.2 kJ Remarks The difference between WAEC and QAEC is the change in the internal energy ΔEint,AEC during this process. 80 •• The PV diagram in Figure 18-25 represents 3.00 mol of an ideal monatomic gas. The gas is initially at point A. The paths AD and BC represent isothermal changes. If the system is brought to point C along the path ABC, find (a) the initial and final temperatures of the gas, (b) the work done by the gas, and (c) the heat absorbed by the gas. Picture the Problem We can use the ideal-gas law to find the temperatures TA and TC. Because the process AB is isobaric, we can find the area under this line geometrically. We can use the expression for the work done during an isothermal expansion to find the work done between B and C and the first law of thermodynamics to find QABC. (a) Using the ideal-gas law, find the temperature at point A: TA = = PAVA nR (4.0 atm )(4.01 L ) (3.00 mol)⎛⎜ 8.206 ×10 − 2 ⎝ L ⋅ atm ⎞ ⎟ mol ⋅ K ⎠ = 65.2 K = 65 K Use the ideal-gas law to find the temperature at point C: TC = = PCVC nR (1.0 atm )(20.0 L ) (3.00 mol)⎛⎜ 8.206 ×10 − 2 ⎝ = 81.2 K = 81 K L ⋅ atm ⎞ ⎟ mol ⋅ K ⎠ Heat and the First Law of Thermodynamics 1845 (b) Express the work done by the gas along the path ABC: WABC = WAB + WBC ⎛V ⎞ = PABΔVAB + nRTB ln⎜⎜ C ⎟⎟ ⎝ VB ⎠ Use the ideal-gas law to find the volume of the gas at point B: VB = nRTB = PB L ⋅ atm ⎞ ⎟ (81.2 K ) mol ⋅ K ⎠ = 5.00 L 4.0 atm (3.00 mol)⎛⎜ 8.206 ×10 − 2 ⎝ Substitute numerical values and evaluate WABC: L ⋅ atm ⎞ ⎛ WABC = (4.0 atm )(5.00 L − 4.01 L ) + (3.00 mol)⎜ 8.206 ×10 − 2 ⎟ mol ⋅ K ⎠ ⎝ ⎛ 20.0 L ⎞ ⎟⎟ × (81.2 K )ln⎜⎜ ⎝ 5.00 L ⎠ = 31.7 L ⋅ atm × (c) Apply the first law of thermodynamics to obtain: 101.325 J = 3.21 kJ = 3.2 kJ atm QABC = WABC + ΔEint = WABC + CV ΔT = WAEC + 32 nRΔT = WAEC + 32 nR(TC − TA ) Substitute numerical values and evaluate QABC: J ⎞ ⎛ QABC = 3.21 kJ + 32 (3.00 mol)⎜ 8.314 ⎟ (81.2 K − 65.2 K ) = 3.8 kJ mol ⋅ K ⎠ ⎝ Remarks: The difference between WABC and QABC is the change in the internal energy ΔEint,ABC during this process. 81 •• The PV diagram in Figure 18-25 represents 3.00 mol of an ideal monatomic gas. The gas is initially at point A. The paths AD and BC represent isothermal changes. If the system is brought to point C along the path ADC, find (a) the initial and final temperatures of the gas, (b) the work done by the gas, and (c) the heat absorbed by the gas. Picture the Problem We can use the ideal-gas law to find the temperatures TA and TC. Because the process DC is isobaric, we can find the area under this line geometrically. We can use the expression for the work done during an isothermal 1846 Chapter 18 expansion to find the work done between A and D and the first law of thermodynamics to find QADC. (a) Using the ideal-gas law, find the temperature at point A: TA = = PAVA nR (4.0 atm )(4.01 L ) (3.00 mol)⎛⎜ 8.206 ×10 − 2 ⎝ L ⋅ atm ⎞ ⎟ mol ⋅ K ⎠ = 65.2 K = 65 K Use the ideal-gas law to find the temperature at point C: TC = = PCVC nR (1.0 atm )(20 L ) (3.00 mol)⎛⎜ 8.206 ×10 − 2 ⎝ L ⋅ atm ⎞ ⎟ mol ⋅ K ⎠ = 81 K (b) Express the work done by the gas along the path ADC: W ADC = W AD + W DC ⎛V ⎞ = nRTA ln⎜⎜ D ⎟⎟ + PDC ΔV DC ⎝ VA ⎠ Use the ideal-gas law to find the volume of the gas at point D: VD = nRTD = PD (3.00 mol)⎛⎜ 8.206 ×10 − 2 ⎝ L ⋅ atm ⎞ ⎟ (65.2 K ) mol ⋅ K ⎠ 1.0 atm = 16.051 L Substitute numerical values and evaluate WADC: ⎛ 16.1 L ⎞ L ⋅ atm ⎞ ⎛ ⎟⎟ WADC = (3.00 mol)⎜ 8.206 ×10 − 2 ⎟ (65.2 K ) ln⎜⎜ mol ⋅ K ⎠ ⎝ ⎝ 4.01 L ⎠ + (1.0 atm )(20.0 L − 16.1 L ) 101.325 J = 26.2 L ⋅ atm × = 2.65 kJ = 2.7 kJ L ⋅ atm Heat and the First Law of Thermodynamics 1847 (c) Apply the first law of thermodynamics to obtain: QADC = WADC + ΔEint = WADC + CV ΔT = WADC + 32 nRΔT = WADC + 32 nR(TC − TA ) Substitute numerical values and evaluate QADC: J ⎞ ⎛ QADC = 2.65 kJ + 32 (3.00 mol)⎜ 8.314 ⎟ (81.2 K − 65.2 K ) = 3.3 kJ mol ⋅ K ⎠ ⎝ 82 •• Suppose that the paths AD and BC in Figure 18-25 represent adiabatic processes. What are the work done by the gas and the heat absorbed by the gas in following the path ABC? Picture the Problem We can use the ideal-gas law to find the temperatures TA and TC. Because the process AB is isobaric, we can find the area under this line geometrically. We can find the work done during the adiabatic expansion between B and C using WBC = −CV ΔTBC and the first law of thermodynamics to find QABC. The work done by the gas along path ABC is given by: WABC = WAB + WBC = PAB ΔVAB − C V ΔTBC = PAB ΔVAB − 32 nRΔTBC because, with Qin = 0, WBC = −ΔEint,BC. Use the ideal-gas law to find TA: TA = = PAVA nR (4.0 atm )(4.01 L ) (3.00 mol)⎛⎜ 8.206 ×10 − 2 ⎝ L ⋅ atm ⎞ ⎟ mol ⋅ K ⎠ = 65.2 K Use the ideal-gas law to express TB: TB = PBVB nR Substitute numerical values and evaluate TB: TB = (4.0 atm )(8.71 L ) (3.00 mol)⎛⎜ 8.206 ×10−2 L ⋅ atm ⎞⎟ ⎝ = 142 K Use the ideal-gas law to express TC: TC = PCVC nR mol ⋅ K ⎠ 1848 Chapter 18 Substitute numerical values and evaluate TC: TC = (1.0 atm )(20.0 L ) (3.00 mol)⎛⎜ 8.206 ×10−2 L ⋅ atm ⎞⎟ mol ⋅ K ⎠ ⎝ = 81.2 K Apply the pressure-volume relationship for a quasi-static adiabatic process to the gas at points B and C to find the volume of the gas at point B: PBVBγ = PCVCγ and 1 3 ⎛ P ⎞γ ⎛ 1.0 atm ⎞ 5 ⎟⎟ (20.0 L ) VB = ⎜⎜ C ⎟⎟ VC = ⎜⎜ ⎝ 4.0 atm ⎠ ⎝ PB ⎠ = 8.71L Substitute numerical values and evaluate WABC: L ⋅ atm ⎞ ⎛ WABC = (4.0 atm )(8.71 L − 4.01 L ) − 32 (3.00 mol)⎜ 8.206 ×10 − 2 ⎟ mol ⋅ K ⎠ ⎝ × (81.2 K − 142 K ) 101.325 J = 41.3 L ⋅ atm × = 4.18 kJ = 4.2 kJ atm Apply the 1st law of thermodynamics to obtain: QABC = WABC + ΔEint = WABC + CV ΔT = WABC + 32 nRΔT = WABC + 32 nR(TC − TA ) Substitute numerical values and evaluate QABC: J ⎞ ⎛ QABC = 4.18 kJ + 32 (3.00 mol)⎜ 8.314 ⎟ (81.2 K − 65.2 K ) = 4.8 kJ mol ⋅ K ⎠ ⎝ 83 •• [SSM] As part of a laboratory experiment, you test the calorie content of various foods. Assume that when you eat these foods, 100% of the energy released by the foods is absorbed by your body. Suppose you burn a 2.50-g potato chip, and the resulting flame warms a small aluminum can of water. After burning the potato chip, you measure its mass to be 2.20 g. The mass of the can is 25.0 g, and the volume of water contained in the can is 15.0 ml. If the temperature increase in the water is 12.5°C, how many kilocalories (1 kcal = 1 dietary calorie) per 150-g serving of these potato chips would you estimate there are? Assume the can of water captures 50.0 percent of the heat released during the burning of the potato chip. Note: Although the joule is the SI unit of choice in most thermodynamic situations, the food industry in the United States currently expresses the energy released during metabolism in terms of the ″dietary calorie,″ which is our kilocalorie. Heat and the First Law of Thermodynamics 1849 Picture the Problem The ratio of the energy in a 150-g serving to the energy in 0.30 g of potato chip is the same as the ratio of the masses of the serving and the amount of the chip burned while heating the aluminum can and the water in it. The ratio of the energy in a 150-g serving to the energy in 0.30 g of potato chip is the same as the ratio of the masses of the serving and the amount of the chip burned while heating the aluminum can and the water in it: Letting f represent the fraction of the heat captured by the can of water, express the energy transferred to the aluminum can and the water in it during the burning of the potato chip: Substituting for Q0.30 g yields and solving for Q150-g serving yields: Q150-g serving Q0.30 g = 150 g = 500 0.30 g or Q150 g serving = 500Q0.30 g fQ0.30 g = QAl + QH 2 O = mAl cAl ΔT + mH 2 O cH 2 O ΔT ( ) = mAl cAl + mH 2 O cH 2 O ΔT where ΔT is the common temperature change of the aluminum cup and the water it contains. Q150-g serving = ( ) 500 mAl cAl + mH 2 O cH 2 O ΔT f Substitute numerical values and evaluate Q150-g serving : Q150-g serving ⎡ ⎛ ⎛ kJ ⎞ kJ ⎞⎤ ⎟⎟ + (0.0150 kg )⎜⎜ 4.184 ⎟⎟⎥ (12.5 C°) 500⎢(0.0250 kg )⎜⎜ 0.900 ⋅ ⋅ kg K kg K ⎝ ⎠ ⎝ ⎠⎦ ⎣ = 0.500 1 cal = 1.07 ×10 6 J × = 256 ×10 3 cal ≈ 256 kcal 4.184 J 84 •• Diesel engines operate without spark plugs, unlike gasoline engines. The cycle that diesel engines undergo involves adiabatically compressing the air in a cylinder, and then fuel is injected. When the fuel is injected, if the air temperature inside the cylinder is above the fuel’s flashpoint, the fuel-air mixture will ignite. Most diesel engines have compression ratios in the range from 14:1 to 25:1. For this range of compression ratios (which are the ratio of maximum to minimum volume), what is the range of maximum temperatures of the air in the cylinder, assuming the air is taken into the cylinder at 35°C? Most modern gasoline engines typically have compression ratios on the order of 8:1. Explain why you expect the diesel engine to require a better (more efficient) cooling system than its gasoline counterpart. 1850 Chapter 18 Picture the Problem You can use the equation-of-state for an adiabatic process to find the range of maximum temperatures of the air in the cylinder. For an adiabatic process from state i to state f: TiVi γ −1 = Tf Vfγ −1 where, for a diatomic gas, γ = 1.4. Solving for Tf yields: ⎛V ⎞ Vi γ −1 Tf = Ti γ −1 = Ti ⎜⎜ i ⎟⎟ Vf ⎝ Vf ⎠ Substitute numerical values and evaluate Tf for an engine with a compression ratio of 14:1: ⎛ 14 ⎞ Tf = (273 K + 35 C°)⎜ ⎟ ⎝1⎠ = 885 K = 612°C Substitute numerical values and evaluate Tf for an engine with a compression ratio of 25:1: ⎛ 25 ⎞ Tf = (273 K + 35 C°)⎜ ⎟ ⎝ 1 ⎠ = 1116 K = 843°C γ −1 1.4−1 1.4−1 The range of maximum temperatures of the air in the cylinder, assuming it is taken into the cylinder at 35°C, is 612°C → 843°C . Gasoline engines, with much lower compression ratios, would clearly have lower maximum operating temperatures and thus be subject to overall, average, lower temperatures and not require as extensive cooling systems. 85 •• At very low temperatures, the specific heat of a metal is given by c = aT + bT3. For copper, a = 0.0108 J/kg⋅K2 and b = 7.62 × 10–4 J/kg⋅K4. (a) What is the specific heat of copper at 4.00 K? (b) How much heat is required to heat copper from 1.00 to 3.00 K? Picture the Problem We can find c at T = 4.00 K by direct substitution. Because c is a function of T, we can integrate dQ over the given temperature interval in order to find the heat required to heat copper from 1.00 to 3.00 K. (a) Substitute for a and b to obtain: ⎛ ⎛ J ⎞ J ⎞ 3 ⎟ T + ⎜⎜ 7.62 ×10 −4 ⎟T c = ⎜⎜ 0.0108 2 ⎟ kg ⋅ K ⎠ kg ⋅ K 4 ⎟⎠ ⎝ ⎝ Heat and the First Law of Thermodynamics 1851 Evaluate c at T = 4.00 K: ⎛ ⎛ J ⎞ J ⎞ 3 ⎟ (4.00 K ) ⎟ (4.00 K ) + ⎜⎜ 7.62 ×10 −4 c (4.00 K ) = ⎜⎜ 0.0108 2 ⎟ 4 ⎟ kg ⋅ K ⎠ kg ⋅ K ⎠ ⎝ ⎝ J = 9.20 × 10 −2 kg ⋅ K (b) The heat required to heat copper from 1.00 to 3.00 K is given by: 3.00 K Q= ∫ c(T ) dT 1.00 K Substituting for c(T) yields: 3.00 K 3.00 K ⎛ ⎛ J ⎞ J ⎞ ⎟ TdT + ⎜⎜ 7.62 ×10 − 4 ⎟ T 3 dT Q = ⎜⎜ 0.0108 2 ⎟ ∫ 4 ⎟ ∫ kg ⋅ K ⎠1.00 K kg ⋅ K ⎠1.00 K ⎝ ⎝ Evaluate this integral to obtain: 3.00 K 3.00 K ⎛ ⎛ J ⎞ ⎡T 2 ⎤ J ⎞ ⎡T 4 ⎤ −4 ⎟⎢ ⎥ ⎟⎢ ⎥ + ⎜ 7.62 × 10 Q = ⎜⎜ 0.0108 = 0.0584 J/kg kg ⋅ K 2 ⎟⎠ ⎣ 2 ⎦ 1.00 K ⎜⎝ kg ⋅ K 4 ⎟⎠ ⎣ 4 ⎦1.00 K ⎝ 86 •• How much work must be done on 30.0 g of carbon monoxide (CO) at standard temperature and pressure to compress it to one-fifth of its initial volume if the process is (a) isothermal, (b) adiabatic? Picture the Problem Let the subscripts 1 and 2 refer to the initial and final state respectively. Because the gas is initially at STP, we know that V1 = 22.4 L/mol, P1 = 1 atm, and T1 = 273 K. We can use W = −nRT ln (V2 V1 ) to find the work done on the gas during an isothermal compression. We can relate the work done on a gas during an adiabatic process to the pressures and volumes of the initial and PV − P V final points on the path using W = 1 1 2 2 and find P1 by eliminating P2 using γ −1 γ γ P1V1 = P2V2 , where, for a diatomic gas, γ = 1.4. See Appendix C for the molar mass of CO. (a) Express the work done on the gas in compressing it isothermally: Because the number of moles of CO is the mass m of CO divided by its molar mass: ⎛V ⎞ Won = −nRT ln⎜⎜ 2 ⎟⎟ ⎝ V1 ⎠ Won = − ⎛V ⎞ m RT ln⎜⎜ 2 ⎟⎟ M ⎝ V1 ⎠ 1852 Chapter 18 Substitute numerical values and evaluate Won: ⎛ 30.0 g ⎞ ⎛ J ⎞ ⎛1⎞ ⎟⎟ ⎜ 8.314 Won = −⎜⎜ ⎟ (273 K )ln⎜ ⎟ = 3.91 kJ mol ⋅ K ⎠ ⎝5⎠ ⎝ 28.01g/mol ⎠ ⎝ (b) Express the work done on the gas in compressing it adiabatically: Won = − P1V1 − P2V2 γ −1 ⎛ P ⎞ P1 ⎜⎜V1 − 2 V2 ⎟⎟ P1 ⎠ =− ⎝ γ −1 Using the equation for a quasi-static adiabatic process, relate the initial and final pressures and volumes: P ⎛V ⎞ PV = P V ⇒ 2 = ⎜⎜ 1 ⎟⎟ P1 ⎝ V2 ⎠ γ 1 1 γ γ 2 2 Substitute for P2/P1and simplify to obtain: γ γ γ ⎞ ⎛ ⎛ ⎛ ⎛ V1 ⎞ ⎛ V1 ⎞ V1 ⎞⎟ ⎛ V1 ⎞ ⎟ ⎜ ⎜ ⎜ P1 V1 − ⎜⎜ ⎟⎟ V2 P1 V1 − ⎜⎜ ⎟⎟ P1V1 1 − 0.2⎜⎜ ⎟⎟ ⎟ ⎜ ⎜ ⎜ V2 ⎠ V2 ⎠ 5 ⎟ ⎝ ⎝ ⎝ V2 ⎠ ⎠ ⎠ ⎝ ⎝ ⎝ =− =− Won = − γ −1 γ −1 γ −1 ⎞ ⎟ ⎟ ⎠ Substitute numerical values and evaluate Won: (101.325 kPa )(1.071mol)⎛⎜ 22.4 W =− ⎝ 1.4 − 1 ( L ⎞ 1.4 ⎟ 1 − 0.2(5) mol ⎠ ) = 5.49 kJ 87 •• How much work must be done on 30.0 g of carbon dioxide (CO2) at standard temperature and pressure to compress it to one-fifth of its initial volume if the process is (a) isothermal, (b) adiabatic? Picture the Problem Let the subscripts 1 and 2 refer to the initial and final state respectively. Because the gas is initially at STP, we know that V1 = 22.4 L/mol, P1 = 1 atm, and T1 = 273 K. We can use W = −nRT ln (V2 V1 ) to find the work done on the gas during an isothermal compression. We can relate the work done on a gas during an adiabatic process to the pressures and volumes of the initial PV − P V and final points on the path using W = 1 1 2 2 and find P1 by eliminating P2 γ −1 γ γ using P1V1 = P2V2 . We can find γ using the data in Table 18-3. See Appendix C for the molar mass of CO2. Heat and the First Law of Thermodynamics 1853 (a) Express the work done on the gas in compressing it isothermally: Because the number of moles of CO2 is the mass m of CO2 divided by its molar mass: ⎛V ⎞ Won = −nRT ln⎜⎜ 2 ⎟⎟ ⎝ V1 ⎠ Won = − ⎛V ⎞ m RT ln⎜⎜ 2 ⎟⎟ M ⎝ V1 ⎠ Substitute numerical values and evaluate Won: ⎛ 30.0 g ⎞ ⎛ J ⎞ ⎛1⎞ ⎟⎟ ⎜ 8.314 Won = −⎜⎜ ⎟ (273 K )ln⎜ ⎟ = 2.49 kJ mol ⋅ K ⎠ ⎝5⎠ ⎝ 44.01g/mol ⎠ ⎝ (b) Express the work done on the gas in compressing it adiabatically: Won = − P1V1 − P2V2 γ −1 ⎛ P ⎞ P1 ⎜⎜V1 − 2 V2 ⎟⎟ P1 ⎠ =− ⎝ γ −1 Using the equation for a quasistatic adiabatic process, relate the initial and final pressures and volumes: P ⎛V ⎞ P1V1 = P2V2 ⇒ 2 = ⎜⎜ 1 ⎟⎟ P1 ⎝ V2 ⎠ γ γ γ Substitute for P2/P1 and simplify to obtain: γ γ γ ⎛ ⎛ ⎞ ⎛ ⎛ V1 ⎞ ⎛ V1 ⎞ V1 ⎞⎟ ⎛ V1 ⎞ ⎜ ⎜ ⎟ ⎜ P1 V1 − ⎜⎜ ⎟⎟ V2 P1 V1 − ⎜⎜ ⎟⎟ P1V1 1 − 0.2⎜⎜ ⎟⎟ ⎜ ⎜ ⎟ ⎜ V2 ⎠ V2 ⎠ 5 ⎟ ⎝ V2 ⎠ ⎝ ⎝ ⎝ ⎠ ⎝ ⎠ ⎝ Won = − =− =− γ −1 γ −1 γ −1 From Table 18-3 we have: cV = 3.39 R and cP = (3.39 + 1.02 )R = 4.41R Evaluate γ: γ = cP 4.41R = = 1.30 cV 3.39 R ⎞ ⎟ ⎟ ⎠ 1854 Chapter 18 Substitute numerical values and evaluate Won: (101.325 kPa )(0.6817 mol)⎛⎜ 22.4 Won = − ⎝ 1.3 − 1 ( L ⎞ 1.30 ⎟ 1 − 0.2(5) mol ⎠ ) = 3.20 kJ 88 •• How much work must be done on 30.0 g of argon (Ar) at standard temperature and pressure to compress it to one-fifth of its initial volume if the process is (a) isothermal, (b) adiabatic? Picture the Problem Let the subscripts 1 and 2 refer to the initial and final states respectively. Because the gas is initially at STP, we know that V1 = 22.4 L/mol, P1 = 1 atm, and T1 = 273 K. We can use W = −nRT ln (V2 V1 ) to find the work done on the gas during an isothermal compression. We can relate the work done on a gas during an adiabatic process to the pressures and volumes of the initial PV − P V and final points on the path using W = 1 1 2 2 and find P1 by eliminating P2 γ −1 γ γ using P1V1 = P2V2 , where, for a monatomic gas, γ = 1.67. See Appendix C for the molar mass of Ar. (a) Express the work done on the gas in compressing it isothermally: Because the number of moles of Ar is the mass m of Ar divided by its molar mass: ⎛V ⎞ Won = −nRT ln⎜⎜ 2 ⎟⎟ ⎝ V1 ⎠ Won = − ⎛V ⎞ m RT ln⎜⎜ 2 ⎟⎟ M ⎝ V1 ⎠ Substitute numerical values and evaluate Won: ⎛ ⎞⎛ 30.0 g J ⎞ ⎛1⎞ ⎟⎟ ⎜ 8.314 Won = −⎜⎜ ⎟ (273 K ) ln⎜ ⎟ = 2.74 kJ mol ⋅ K ⎠ ⎝5⎠ ⎝ 39.948 g/mol ⎠ ⎝ (b) Express the work done on the gas in compressing it adiabatically: Won = − P1V1 − P2V2 γ −1 ⎛ P ⎞ P1 ⎜⎜V1 − 2 V2 ⎟⎟ P1 ⎠ =− ⎝ γ −1 Using the equation for a quasi-static adiabatic process, relate the initial and final pressures and volumes: P ⎛V ⎞ P1V1 = P2V2 ⇒ 2 = ⎜⎜ 1 ⎟⎟ P1 ⎝ V2 ⎠ γ γ γ Heat and the First Law of Thermodynamics 1855 Substitute for P2/P1 and simplify to obtain: γ γ γ ⎛ ⎞ ⎛ ⎛ ⎛ V1 ⎞ ⎛ V1 ⎞ V1 ⎞⎟ ⎛ V1 ⎞ ⎜ ⎟ ⎜ ⎜ P1 V1 − ⎜⎜ ⎟⎟ V2 P1 V1 − ⎜⎜ ⎟⎟ P1V1 1 − (0.2)⎜⎜ ⎟⎟ ⎜ ⎟ ⎜ ⎜ V2 ⎠ V2 ⎠ 5 ⎟ ⎝ ⎝ ⎝ V2 ⎠ ⎝ ⎠ ⎝ ⎠ ⎝ Won = − =− =− γ −1 γ −1 γ −1 ⎞ ⎟ ⎟ ⎠ Substitute numerical values and evaluate Won: (101.325 kPa )(0.75098 mol)⎛⎜ 22.4 ⎝ 1.67 − 1 Won = − ( L ⎞ 1.67 ⎟ 1 − (0.2 )(5) mol ⎠ ) = 4.93 kJ 89 •• [SSM] A thermally insulated system consists of 1.00 mol of a diatomic gas at 100 K and 2.00 mol of a solid at 200 K that are separated by a rigid insulating wall. Find the equilibrium temperature of the system after the insulating wall is removed, assuming that the gas obeys the ideal-gas law and that the solid obeys the Dulong–Petit law. Picture the Problem We can use conservation of energy to relate the equilibrium temperature to the heat capacities of the gas and the solid. We can apply the Dulong-Petit law to find the heat capacity of the solid at constant volume and use the fact that the gas is diatomic to find its heat capacity at constant volume. Apply conservation of energy to this process: ΔQ = Qgas + Qsolid = 0 Use Q = C V ΔT to substitute for Qgas and Qsolid: C V,gas (Tequil − 100 K ) + C V,solid (Tequil − 200 K ) = 0 Solving for Tequil yields: Tequil = (100 K )(CV,gas ) + (200 K )(CV,solid ) Using the Dulong-Petit law, determine the heat capacity of the solid at constant volume: C V,solid = 3nsolid R The heat capacity of the gas at constant volume is given by: C V,gas = 52 ngas R C V,gas + C V,solid 1856 Chapter 18 Substitute for CV,solid and CV,gas and simplify to obtain: Tequil = (100 K ) (52 ngas R ) + (200 K )(3nsolid R ) (100 K )(52 ngas ) + (200 K )(3nsolid ) 5 2 ngas R + 3nsolid R = 5 2 ngas + 3nsolid Substitute numerical values for ngas and nsolid and evaluate Tequil: Tequil = (100 K )( 52 )(1.00 mol) + (200 K )(3)(2.00 mol) = 5 ( ) ( ) 2 1.00 mol + 3 2.00 mol 171 K 90 •• When an ideal gas undergoes a temperature change at constant volume, its internal energy change is given by the formula ΔEint = CvΔT. However, this formula correctly gives the change in internal energy whether the volume remains constant or not. (a) Explain why this formula gives correct results for an ideal gas even when the volume changes. (b) Using this formula, along with the first law of thermodynamics, show that for an ideal gas Cp = Cv + nR. Picture the Problem (a) ΔEint = C V ΔT correctly gives the change in internal energy when the temperature changes and the volume is not constant because ΔEint is the same for all gas processes that have the same ΔT. For an ideal gas, the internal energy is the sum of the kinetic energies of the gas molecules and this sum is proportional to kT. Any two processes that change the thermal energy of the gas by ΔEint will cause the same temperature change ΔT. Consequently, ΔEint is independent of the process that takes the gas from one state to another. (b) Use the first law of thermodynamics to relate the work done on the gas, the heat entering the gas, and the change in the internal energy of the gas: ΔEint = Qin + Won Substituting for ΔEint yields: C V ΔT = Qin + Won The work done on the gas during a constant-pressure process is given by: Won = − PΔV = − P(Vf − Vi ) Heat and the First Law of Thermodynamics 1857 Use the ideal gas law to substitute for Vf and Vi and then simplify to obtain: ⎛ nRTf nRTi − Won = − P⎜ P ⎝ P = − nRΔT ⎞ ⎟ = − nR (Tf − Ti ) ⎠ Substituting for Won gives: C V ΔT = Qin − nRΔT Solve for Qin and simplify to obtain: Qin = C V ΔT + nRΔT = (C V + nR )ΔT = C P ΔT where C P = C V + nR 91 •• An insulated cylinder is fitted with an insulated movable piston to maintain constant pressure. The cylinder initially contains 100 g of ice at –10ºC. Heat is transferred to the ice at a constant rate by a 100-W heater. Make a graph showing the temperature of the ice/water/ steam as a function of time starting at ti when the temperature is –10ºC and ending at tf when the temperature is 110ºC. Picture the Problem Knowing the rate at which energy is supplied, we can obtain the data we need to plot this graph by finding the time required to warm the ice to 0°C, melt the ice, warm the water formed from the ice to 100°C, vaporize the water, and warm the water to 110°C. Find the time required to warm the ice to 0°C: Δt1 = mcice ΔT = P (0.100 kg )⎛⎜⎜ 2.0 ⎝ Find the time required to melt the ice: kJ ⎞ ⎟ (0°C − (− 10°C )) kg ⋅ K ⎟⎠ = 20.0 s ≈ 0.3 min J 100 s Δt 2 = mLf = P (0.100 kg )⎛⎜⎜ 333.5 kJ ⎞⎟⎟ kg ⎠ ⎝ 100 J s = 333.5 s ≈ 5.6 min Find the time required to heat the water to 100°C: Δt3 = mcw ΔT P (0.100 kg )⎛⎜⎜ 4.18 kJ ⎞ ⎟ (100°C ) kg ⋅ K ⎟⎠ ⎝ = J 100 s = 418 s ≈ 7.0 min 1858 Chapter 18 Find the time required to vaporize the water: Δt 4 = mLV = P (0.100 kg )⎛⎜⎜ 2257 kJ ⎞⎟⎟ kg ⎠ ⎝ 100 J s = 2257 s ≈ 37.6 min Find the time required to heat the vapor to 110°C: Δt 5 = mcsteam ΔT P (0.100 kg )⎛⎜⎜ 2.0 kJ ⎞ ⎟ (10°C ) kg ⋅ K ⎟⎠ ⎝ = J 100 s = 20.0 s ≈ 0.3 min A graph of T as a function of time follows: T , °C 110 100 0 −10 0.3 5.9 12.9 50.5 50.8 t , min 92 •• (a) In this problem, 2.00 mol of a diatomic ideal gas expand adiabatically and quasi-statically. The initial temperature of the gas is 300 K. The work done by the gas during the expansion is 3.50 kJ. What is the final temperature of the gas? (b) Compare your result to the result you would get if the gas were monatomic. Picture the Problem We know that, for an adiabatic process, Qin = 0. Hence the work done by the expanding gas equals the change in its internal energy. Because we’re given the work done by the gas during the expansion, we can express the change in the temperature of the gas in terms of this work and CV. Heat and the First Law of Thermodynamics (a) Express the final temperature of the gas as a result of its expansion: Tf = Ti + ΔT Apply the equation for adiabatic work and solve for ΔT: Wadiabatic = −CV ΔT and ΔT = − Substitute for ΔT to obtain: Substitute numerical values and evaluate Tf for a diatomic gas: 1859 Wadiabatic W = − 5adiabatic CV 2 nR Tf = Ti − Wadiabatic 5 2 nR Tf = 300 K − 3.50 kJ J ⎞ ⎛ 5 ⎟ 2 (2.00 mol )⎜ 8.314 mol ⋅ K ⎠ ⎝ = 216 K (b) Because a monatomic gas has only 3 degrees of freedom: C V = 32 nR and Tf = Ti − Substitute numerical values and evaluate Tf for a monatomic gas: Wadiabatic 3 2 nR Tf = 300 K − 3.50 kJ J ⎞ ⎛ 3 ⎟ 2 (2.00 mol )⎜ 8.314 mol ⋅ K ⎠ ⎝ = 160 K 93 •• A vertical insulated cylinder is divided into two parts by a movable piston of mass m. The piston is initially held at rest. The top part is evacuated and the bottom part is filled with 1.00 mol of diatomic ideal gas at temperature 300 K. After the piston is released and the system comes to equilibrium, the volume occupied by gas is halved. Find the final temperature of the gas. Picture the Problem Let the subscripts 1 and 2 refer to the initial and final states in this adiabatic expansion. We can use an equation describing a quasi-static adiabatic process to express the final temperature as a function of the initial temperature and the initial and final volumes. 1860 Chapter 18 Using the equation for a quasi-static adiabatic process, relate the initial and final volumes and temperatures: T2V2γ −1 = T1V1γ −1 Solve for T2 and simplify to obtain: ⎛V ⎞ T2 = T1 ⎜⎜ 1 ⎟⎟ ⎝ V2 ⎠ Substitute the numerical value for T1 and evaluate T2: T2 = (300 K )(2 ) γ −1 ⎛ V ⎞ = T1 ⎜⎜ 1 1 ⎟⎟ ⎝ 2 V1 ⎠ 1.4−1 γ −1 = T1 (2 ) γ −1 = 396 K According to the Einstein model of a crystalline solid, the internal 3 N kT energy per mole is given by U = TE /AT E where TE is a characteristic temperature −1 e called the Einstein temperature, and T is the temperature of the solid in kelvins. Use this expression to show that a crystalline solid’s molar heat capacity at 2 e TE T ⎛ TE ⎞ constant volume is given by c' V = 3R⎜ ⎟ . 2 ⎝ T ⎠ e TE T − 1 94 ••• ( ) Picture the Problem The molar heat capacity at constant volume is related to the 1 dU internal energy per mole according to c' v = . We can differentiate U with n dT respect to temperature and use nR = Nk or R = NAk to establish the result given in the problem statement. 3N A kTE eTE T − 1 From the Einstein model of a crystalline solid, the internal energy per mole is given by: U= Relate the molar heat capacity at constant volume to the internal energy per mol: c' V = Use c' V = c' V = 1 dU n dT 1 dU to express c'V : n dT ⎤ d T ⎡ 1 d ⎡ 3N A kTE ⎤ −1 d ⎡ 1 ⎤ = 3RTE = 3RTE ⎢ eE ⎥ TE T TE T 2 ⎢ ⎥ ⎢ ⎥ T T E n dT ⎣ e dT ⎣ e −1 ⎦ − 1⎦ − 1 ⎥⎦ dT ⎢⎣ e ( ) 2 ⎤ ⎡ T T ⎛ TE ⎞⎤ ⎡ −1 eTE T ⎛ TE ⎞ E = 3RTE ⎢ − = 3 R e ⎜ ⎟ ⎜ ⎟ ⎥ 2 ⎢ 2 ⎥ 2 T T ⎝ T ⎠⎦ ⎝ T ⎠ eTE T − 1 ⎢⎣ e E − 1 ⎥⎦ ⎣ ( ) ( ) ( T ) −1 Heat and the First Law of Thermodynamics 1861 95 ••• [SSM] (a) Use the results of Problem 94 to show that in the limit that T >> TE, the Einstein model gives the same expression for specific heat that the Dulong–Petit law does. (b) For diamond, TE is approximately 1060 K. Integrate numerically dEint = cv′ dT to find the increase in the internal energy if 1.00 mol of diamond is heated from 300 to 600 K. Picture the Problem (a) We can rewrite our expression for c'V by dividing its numerator and denominator by eTE T and then using the power series for ex to show that, for T > TE, c'V ≈ 3R . In part (b), we can use the result of Problem 108 to obtain values for c'V every 100 K between 300 K and 600 K and use this data to find ΔU numerically. 2 (a) From Problem 94 we have: eTE T ⎛T ⎞ c' V = 3R⎜ E ⎟ ⎝ T ⎠ e TE T − 1 ( Divide the numerator and denominator by eTE T to obtain: ) 2 ⎛T ⎞ c' V = 3R⎜ E ⎟ 2TE ⎝T ⎠ e 2 ⎛T ⎞ = 3R⎜ E ⎟ TE ⎝T ⎠ e T T 2 1 − 2eTE eTE T T 1 − 2 + e −TE +1 T Express the exponential terms in their power series to obtain: 2 e TE T −2+e −TE T 2 T T 1⎛T ⎞ 1⎛T ⎞ = 1 + E + ⎜ E ⎟ + ... − 2 + 1 − E + ⎜ E ⎟ + ... T 2⎝ T ⎠ T 2⎝ T ⎠ 2 ⎛T ⎞ ≈ ⎜ E ⎟ for T >> TE ⎝T ⎠ Substitute for e TE obtain: T − 2 + e −TE T to 2 1 ⎛T ⎞ = 3R c' V ≈ 3R⎜ E ⎟ 2 ⎝ T ⎠ ⎛ TE ⎞ ⎜ ⎟ ⎝T ⎠ (b) Use the result of Problem 94 to verify the following table: T cV (K) 300 400 500 600 (J/mol⋅K) 9.65 14.33 17.38 19.35 1862 Chapter 18 The following graph of specific heat as a function of temperature was plotted using a spreadsheet program: 21 19 C V (J/mol-K) 17 15 13 11 9 7 5 300 350 400 450 500 550 600 T (K) Integrate numerically, using the formula for the area of a trapezoid, to obtain: ΔU = 1 2 (1.00 mol)(100 K )(9.65 + 14.33) J mol ⋅ K + 12 (1.00 mol)(100 K )(14.33 + 17.38) J mol ⋅ K + 12 (1.00 mol)(100 K )(17.38 + 19.35) J mol ⋅ K = 4.62 kJ 96 ••• Use the results of the Einstein model in Problem 94 to determine the molar internal energy of diamond (TE = 1060 K) at 300 K and 600 K, and thereby the increase in internal energy as diamond is heated from 300 K to 600 K. Compare your result to that of Problem 95. Picture the Problem We can simplify our calculations by relating Avogadro’s number NA, Boltzmann’s constant k, the number of moles n, and the number of molecules N in the gas and solving for NAk. We can then calculate U300 K, U600 K, and their difference. Heat and the First Law of Thermodynamics Express the increase in internal energy per mole resulting from the heating of diamond: ΔU = U 600 K − U 300 K Express the relationship between Avogadro’s number NA, Boltzmann’s constant k, the number of moles n, and the number of molecules N in the gas: nR = Nk ⇒ R = Substitute in the given equation to obtain: U= 1863 (1) N k = NAk n 3RTE eTE T − 1 Determine U300 K: U 300 K J ⎞ ⎛ 3⎜ 8.314 ⎟ (1060 K ) mol ⋅ K ⎠ ⎝ = e1060 K 300 K − 1 = 795.4 J = 795 J Determine U600 K: U 600 K J ⎞ ⎛ 3⎜ 8.314 ⎟ (1060 K ) mol ⋅ K ⎠ ⎝ = e1060 K 600 K − 1 = 5.4498 kJ = 5.45 kJ Substituting in equation (1) yields: ΔU = U 600 K − U 300 K = 5.4498 kJ − 795.4 J = 4.65 kJ This result agrees with the result of Problem 95 to within 1%. 97 ••• During an isothermal expansion, an ideal gas at an initial pressure P0 expands until its volume is twice its initial volume V0. (a) Find its pressure after the expansion. (b) The gas is then compressed adiabatically and quasi-statically until its volume is V0 and its pressure is 1.32P0. Is the gas monatomic, diatomic, or polyatomic? (c) How does the translational kinetic energy of the gas change in each stage of this process? Picture the Problem The isothermal expansion followed by an adiabatic compression is shown on the PV diagram. The path 1→2 is isothermal and the path 2→3 is adiabatic. We can apply the ideal-gas law for a fixed amount of gas and an isothermal process to find the pressure at point 2 and the pressure-volume relationship for a quasi-static adiabatic process to determineγ. 1864 Chapter 18 (a) Relate the initial and final pressures and volumes for the isothermal expansion and solve for and evaluate the final pressure: P1V1 = P2V2 and V V P2 = P1 1 = P0 1 = V2 2V1 (b) Relate the initial and final pressures and volumes for the adiabatic compression: P2V2γ = P3V3γ 1 2 P0 or γ γ 1 2 P0 (2V0 ) = 1.32 P0V0 which simplifies to 2γ = 2.64 Take the natural logarithm of both sides of this equation and solve for and evaluate γ : ln 2.64 = 1.40 ln 2 and the gas is diatomic . γ ln 2 = ln 2.64 ⇒ γ = (c) During the isothermal process, T is constant and the translational kinetic energy of the gas is unchanged. During the adiabatic process, T3 = 1.32T0, and the translational kinetic energy of the gas increased by a factor of 1.32. 98 ••• If a hole is punctured in a tire, the gas inside will gradually leak out. Assume the following: the area of the hole is A; the tire volume is V; and the time, τ, it takes for most of the air to leak out of the tire can be expressed in terms of the ratio A/V, the temperature T, the Boltzmann constant k, and the initial mass m of the gas molecules inside the tire. (a) Based on these assumptions, use dimensional analysis to find an estimate for τ. (b) Use the result of Part (a) to estimate the time it takes for a car tire with a nail hole punched in it to go flat. Picture the Problem In (a) we’ll assume that τ = f (A/V, T, k, m) with the factors dependent on constants a, b, c, and d that we’ll find using dimensional analysis. In (b) we’ll use our result from (a) and assume that the diameter of the puncture is Heat and the First Law of Thermodynamics 1865 about 2 mm, that the tire volume is 0.1 m3, and that the air temperature is 20°C. (a) Express τ = f (A/V, T, k, m): ⎛ A⎞ τ = ⎜ ⎟ (T ) b (k ) c (m ) d ⎝V ⎠ Rewrite this equation in terms of the dimensions of the physical quantities to obtain: ⎛ ML2 ⎞ d T = (L ) (K ) ⎜⎜ 2 ⎟⎟ (M ) ⎝T K⎠ where K represents the dimension of temperature. Simplify this dimensional equation to obtain: T1 = L− a K b M c L2c K − c T -2c M d or T1 = L2 c−a K b−c M c+d T −2c Equate exponents to obtain: T : − 2c = 1 , L : 2c − a = 0 , K : b − c = 0, and M: c+d = 0 Solve these equations simultaneously to obtain: a = −1 , b = − 12 , c = − 12 , and d = Substituting in equation (1) yields: ⎛ A⎞ τ =⎜ ⎟ ⎝V ⎠ a (1) c 1 −a −1 b (T )− (k )− (m ) 1 2 1 2 1 2 = (b) Substitute numerical values and evaluate τ: τ= π 4 (1.293 kg/m )(0.1m ) 0 .1 m 3 (2 ×10 −3 3 m ) 2 3 (8.314 J/mol ⋅ K )(293 K ) = 232 s ≈ 4 min 1 2 V m A kT 1866 Chapter 18




