Batteries Three Main Components of Batteries Common Dry Cell
advertisement

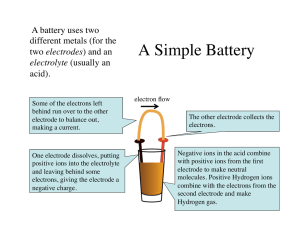
Keys to assembly of an electrolytic cell battery Batteries How Batteries Work Three Main Components of Batteries • Negative terminal (anode): an electrode made of a metal such as zinc that accumulates negative charge ( gains electrons) • Positive terminal (cathode): an electrode made of metal such as copper that accumulates positive charge (loses electrons) • Electrolyte: a liquid solution or paste whose molecules spontaneously seperate into positively or negatively charged atoms or groups of atoms, called ions. • Two metal strips (electrodes) put into a container • Container filled with an electrolyte (liquid solution that separates positive and negative ions) • The electrodes (metal strips) can not touch each other. How does the battery work? • Oxidation-reduction reaction takes place • One electrode accumulates electrons (negative) and the other electrode loses electrons (positive) • The process of separating and accumulating electrons produces the electricity of the battery Common Dry Cell Battery 1 Create PDF files without this message by purchasing novaPDF printer (http://www.novapdf.com)