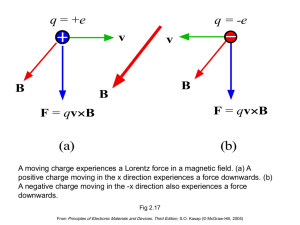
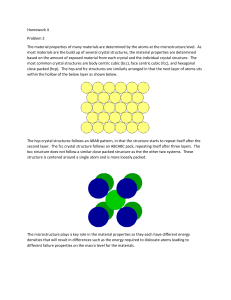
EE 3219 (Chapter 1 Kasap)
advertisement

2006. Fall Semester: Introduction to Electronic Materials & Devices
(Prof. Sin-Doo Lee, Rm. 301-1109, http://mipd.snu.ac.kr
Principles of Electronic Materials and Devices
S. O. Kasap (McGraw Hill, New York, 2000)
Week
Sep. 4 - 6
Sep. 11 - 13
Sep. 25
Sep. 26 - 28
Oct. 2 - 4
Chapter
Chap. 1: Bonding & Solids
Crystalline State
Chap. 2: Drude Model
Electrical Conductivity
Week
Chapter
Nov. 6 - 8
Chap. 5: Extrinsic Semiconductor
Nov. 13 - 15
Nov. 20 - 22
Chap. 6 Semiconductor Devices
Nov. 27 - 29
Chap. 7: Dielectric Materials
Chap. 3: Elementary QM
Dec. 4 - 6
Oct. 9 - 11
Chap. 4: Energy Bands
Oct. 16 - 18
Midterm Exam (18)
Oct. 23 - 25
Chap. 4: Metals, Phonons
Oct. 30
Chap. 5: Intrinsic Semiconductor
Charge Carriers
Dec. 11 - 13
Chap. 8: Superconductivity
Chap. 9: Optical Properties
Final Exam (13)
Chap. 1. Elementary Materials : Science Concepts
1.1 Atomic Structure
- Shell model based on "the Bohr model":
L shell with
two subshells
Nucleus
1s
K
L
2s
2p
1s22s22p2 or [He]2s22p2
Fig. 1.1: The shell model of the atom in which the electrons are
confined to live within certain shells and in subshells within shells.
From Principles of Electronic Materials and Devices, Second Edition, S.O. Kasap (© McGraw-Hill, 2002)
http://Materials.Usask.Ca
1.2 Bonding and Types of Solids
1.2.1 Molecules and General Bonding Principles
- Net force = attractive and repulsive
r=∞
ro
Molecule
Interatomic separation, r
FR = Repulsive force
(a) Force vs r
Repulsion
FN = Net force
Potential Energy, E(r)
Attraction
FA = Attractive force
ro
0
Repulsion
Force
+
Separated atoms
ER = Repulsive PE
E = Net PE
r
0
Attraction
+
Eo
ro
EA = Attractive PE
(b) Potential energy vs r
Fig. 1.3: (a) Force vs interatomic separation and (b) Potential energy vs
interatomic separation.
From Principles of Electronic Materials and Devices, Second Edition, S.O. Kasap (© McGraw-Hill, 2002)
http://Materials.Usask.Ca
1.2.2 Covalently Bonded Solids: H2, CH4, diamond
Covalent bond
H
H
C
H
H
L shell
C
covalent
bonds
K shell
H
H
H
H
(b)
(a)
109.5°
H
H
C
H
H
(c)
Fig. 1.5: (a) Covalent bonding in methane, CH 4 , involves four
hydrogen atoms sharing electrons with one carbon atom. Each
covalent bond has two shared electrons. T he four bonds are
identical and repel each other. (b) Schematic sketch of CH 4 on
paper. (c) In three dimensions, due to symmetry, the bonds are
directed tow ards the corners of a tetrahedron.
From P rinciples of Electronic Materials and D evices, S econ d E dition, S .O . Kasap (© McGraw-Hill, 2002)
http://Materials.U sask.Ca
1.2.3 Metallic Bonding: electron gas or cloud (collective sharing of electrons)
Positive metal
ion cores
Free valence
electrons forming an
electron gas
Fig. 1.7: In metallic bonding the valence electrons from the metal
atoms form a "cloud of electrons" which fills the space between the
metal ions and "glues" the ions together through the coulombic
attraction between the electron gas and positive metal ions.
From Principles of Electronic Materials and Devices, Second Edition, S.O. Kasap (© McGraw-Hill, 2002)
http://Materials.Usask.Ca
1.2.4 Ionically Bonded Solids: salt (cation-anion)
Cl
Na
3s
3s 3p
Closed K and L shells
Closed K and L shells
(a)
Cl-
ClNa+
FA
FA
3s 3p
Na+
r
ro
(b)
(c)
Fig. 1.8: The formation of an ionic bond between Na and Cl atoms
in NaCl. The attraction is due to coulombic forces.
From Principles of Electronic Materials and Devices, Second Edition, S.O. Kasap (© McGraw-Hill, 2002)
http://Materials.Usask.Ca
6
Cl
0
-
-6
-6.3
Cl
-
r=∞
+
Na
1.5 eV
0.28 nm
Cohesive energy
Potential energy E(r), eV/(ion-pair)
- Potential energy per ion pair in solid NaCl
Cl
r=∞
Separation, r
Na
+
Na
ro = 0.28 nm
Fig. 1.10: Sketch of the potential energy per ion-pair in solid NaCl.
Zero energy corresponds to neutral Na and Cl atoms infinitely
separated.
From Principles of Electronic Materials and Devices, Second Edition, S.O. Kasap (© McGraw-Hill, 2002)
http://Materials.Usask.Ca
1.2.5 Secondary Bonding : hydrogen bonds (polar), van der Waals bonds (induced dipolar)
H
O
H
(a)
(b)
Fig. 1.12: The origin of van der Waals bonding between
water molecules. (a) The H2O molecule is polar and has a
net permanent dipole moment. (b) Attractions between the
various dipole moments in water gives rise to van der Waals
bonding.
From Principles of Electronic Materials and Devices, Second Edition, S.O. Kasap (© McGraw-Hill, 2002)
http://Materials.Usask.Ca
Time averaged electron (negative charge)
distribution
Closed L Shell
Ne
Instantaneous electron (negative charge)
distribution fluctuates about the nucleus.
Ionic core
(Nucleus + K-shell)
B
A
van der Waals force
Synchronized fluctuations
of the electrons
Fig. 1.13: Induced dipole-induced dipole interaction and the resulting
van der Waals force.
From Principles of Electronic Materials and Devices, Second Edition, S.O. Kasap (© McGraw-Hill, 2002)
http://Materials.Usask.Ca
1.3 Kinetic Molecular Theory
1.3.1 Mean Kinetic Energy and Temperature
- Gas pressure: the collisions between the gas molecules and the walls of the container
Square Container
Area A
a
Face B
Face A
vy
vx
Gas atoms
a
a
Fig.1.15: The gas molecules in the container are in random motion.
From Principles of Electronic Materials and Devices, Second Edition, S.O. Kasap (© McGraw-Hill, 2002)
http://Materials.Usask.Ca
- The change in the momentum: and
Force = the change of momentum:
Pressure
- For any molecule, , kinetic energy
then
- Heat capacity of one mole of monoatomic "gas" (not solid) at constant volume:
* Maxwell's principle of equipartition of energy - an average of to each degree of
freedom (each degree of freedom has an average energy of and thus the everage
kinetic energy of a monoatomic molecule is × )
1.4, 1.5, 1.6 : Reading Assignment
1.7 The Crystalline State
1.7.1 Type of Crystals : periodic array of points in space - lattice
Crystal
Lattice
Basis
a
a
90°
Unit cell
Unit cell
(a)
(c)
(b)
Basis placement in unit cell
(d)
(0,0)
y
(1/2,1/2)
x
Fig. 1.70: (a) A simple square lattice. The unit cell is a square with a
side a. (b) Basis has two atoms. (c) Crystal = Lattice + Basis. The
unit cell is a simple square with two atoms. (d) Placement of basis
atoms in the crystal unit cell.
UNIT CELL GEOMETRY
CUBIC SYSTEM
a = b = c α = β = γ = 90°
M any metals, Al, Cu, Fe, Pb. Many ceramics and
semiconductors, NaCl, CsCl, LiF, Si, GaAs
Sim ple cubic
Body centered
cubic
Face centered
cubic
TETRAGONAL SYSTEM
a = b - c α = β = γ = 90°
Body centered
tetragonal
Sim ple
tetragonal
In, Sn, Barium Titanate, TiO 2
ORTHORHOMBIC SYSTEM
a - b - c α = β = γ = 90°
S, U, Pl, Ga (<30°C), Iodine, Cementite
(Fe3 C), Sodium Sulfate
Simple
orthorhom bic
Body centered
orthorhombic
Base centered
orthorhom bic
Face centered
orthorhom bic
HEXAGONAL SYSTEM
a = b - c α = β = 90° ; γ = 120°
RHOMBOHEDRAL SYSTEM
a = b = c α = β = γ - 90°
Cadmium, Magnesium, Zinc,
Graphite
Arsenic, Boron, Bismuth, Antimony, M ercury
(<-39°C)
Hexagonal
Rhom bohedral
M ONOCLINIC SYSTEM
a - b - c α = β = 90° ; γ - 90°
TRICLINIC SYSTEM
a - b - c α - β - γ - 90°
α−Selenium, Phosphorus
Potassium dicromate
Lithium Sulfate
Tin Fluoride
Sim ple
m onoclinic
Base centered
m onoclinic
Triclinic
Fig. 1.71: The seven crystal system s (unit cell geometries) and fourteen Bravais
lattices.
- Face-Centered Cubic (FCC) Structure:
FCC Unit Cell
(a)
2R
a
a
a
(b)
a
(c)
Fig. 1.30: (a) The crystal structure of copper is Face Centered Cubic
(FCC). The atoms are positioned at well defined sites arranged periodically
and there is a long range order in the crystal. (b) An FCC unit cell with
closed packed spheres. (c) Reduced sphere representation of the FCC unit
cell. Examples: Ag, Al, Au, Ca, Cu, γ-Fe (>912°C), Ni, Pd, Pt, Rh
- Body-Centered Cubic (BCC) Structure:
a
a
b
Examples: Alkali metals (Li, Na, K, Rb), Cr,
Mo, W, Mn, α-Fe (< 912°C), β-Ti (> 882°C).
Fig. 1.31: Body centered cubic (BCC) crystal structure. (a) A BCC
unit cell with closely packed hard spheres representing the Fe
atoms. (b) A reduced-sphere unit cell.
From Principles of Electronic Materials and Devices, Second Edition, S.O. Kasap (© McGraw-Hill, 2002)
http://Materials.Usask.Ca
- Hexagonal Closed Packed (HCP) Structure:
Layer B
Layer A
Layer B
Layer A
Layer A
(a)
Layer A
(b)
c
a
(c)
(d)
Examples: Be, Mg, α-Ti ( < 882°C ), Cr, Co, Zn, Zr, Cd
Fig. 1.32: The Hexagonal Close Packed (HCP) Crystal Structure. (a) The
Hexagonal Close Packed (HCP) Structure. A collection of many Zn atoms.
Color difference distinguishes layers (stacks). (b) The stacking sequence of
closely packed layers is ABAB (c) A unit cell with reduced spheres (d) The
smallest unit cell with reduced spheres.
- Diamond & Zinc Blende Cubic Structure:
C
S
a
a
Zn
a
a
Fig. 1.33: The diamond unit cell is cubic. The cell has eight
atoms. Grey Sn (α-Sn) and the elemental semiconductors
Ge and Si have this crystal structure.
a
a
Fig. 1.34: The Zinc blende (ZnS) cubic crystal structure. Many
important compound crystals have the zinc blende structure.
Examples: AlAs, GaAs, GaP, GaSb, InAs, InP, InSb, ZnS,
ZnTe.
From Principles of Electronic Materials and Devices, Second Edition, S.O. Kasap (© McGraw-Hill, 2002)
http://Materials.Usask.Ca
Table 1.3 Properties of some important crystal structures
Crystal
Structure
Coordination Number of Atomic
a and R
(R is the radius Number (CN) atoms per Packing
unit cell
Factor
of the atom).
Examples
Simple
cubic
a = 2R
6
1
0.52
None
BCC
a = 4R/√3
8
2
0.68
M any metals: α -Fe, Cr, M o, W
FCC
a = 4R/√2
12
4
0.74
M any metals Ag, Au, Cu, Pt
HCP
a = 2R
c = 1.633a
12
2
0.74
M any metals: Co, M g, Ti, Zn
Diamond
a = 8R/√3
4
8
0.34
Covalent solids:
Diamond, Ge, Si, α -Sn.
Zinc blende
4
8
0.34
M any covalent and ionic solids. M any compund
semiconductors. ZnS, GaAs, GaSb, InAs, InSb
NaCl
6
4 cations
0.67
Ionic solids such as NaCl, AgCl, LiF M gO, CaO
4 anions
(NaCl)
Ionic packing factor depends on relative sizes of
ions.
CsCl
8
1 cation
1 anion
Ionic solids such as CsCl, CsBr, CsI
From Principles of Electronic Materials and Devices, Second Edition, S.O. Kasap (© McGraw-Hill, 2002)
http://Materials.Usask.Ca
1.7.2 Crystal Directions and Planes
z
z
Unit Cell Geometry
Unit cell
c
c β
O
zo
α
b
γ
b
a
[121
P
c
y
xo
a
a
yo
y
b
x
x
(a ) A p a ra lle le p ip e d is ch o se n to d e s crib e g e o m e try o f
a u n it ce ll. W e lin e th e x , y a n d z a x e s w ith th e e d g e s o f
th e p a ra lle le p ip e d tak in g lo w e r-le ft re a r co rn e r a s th e
o rig in
(b ) Id e n tific a tio n o f a d ire ctio n in a c rysta l
[001]
[111]
[010]
-y
[010]
[100]
[111]
[110]
[110]
[111]
-a
x
y
a
[111]
[111]
(c) D ire ctio n s in cu b ic c rysta l syste m
[111]
[111]
[111]
[111]
Family of <111> directions
F ig. 1.39
F rom P rinc iple s of E le ctro nic M ateria ls a nd D e vice s, S ec ond E ditio n, S .O . K asap (© M c G ra w -H ill, 20 02)
http://M a te ria ls.U s as k .C a
- Directions of [uvw]
Family of directions <100>; [100], [010], [001], [
00], [0
0], [00
]
- Planes of (hkl): Miller index, 1/intercept for each axis
Family of planes {100}; (100), (010), (001), (
00), (0
0), (00
)
z
z intercept at ∞
b
Miller Indices (hkz) :
x intercept at a/
1
1
1/ 1
2
c
1
∞
(210)
2
y
a
Unit cell
x
y intercept at b
(a) Identification of a plane in a crystal
z
(010)
(010)
z
(010)
(010)
(010)
y
y
x
x
(110)
(001)
(100)
z
(111)
z
(111)
(110)
y
x
-z
(b) Various planes in the cubic lattice
-y
y
x
Fig. 1.40: L abelling of crystal planes and typical exam ples in the
cubic lattice
From P rinciples of E lectron ic M ateria ls and D e vices, S e cond E d ition , S .O . K asap (© M cG ra w -H ill, 2002 )
http://M a te ria ls.U s ask.C a
1.7.3 Three Phases of Carbon
Covalently bonded layer
Layers bonded by van der Waal
bonding
Cubic crystal
Covalently bonded
layer
Covalently bonded
network of atoms
Hexagonal unit cell
(a) Diamond unit cell
The FCC unit cell of the
Buckminsterfullerene crystal. Each
lattice point has a C60 molecule
(b) Graphite
Buckminsterfullerene (C60) molecule (the
"buckyball" molecule)
(c) Buckminsterfullerene
Fig. 1.42: The three allotropes of carbon.
1.8 Crystalline Defects
1.8.1 Point Defects: vacancy concentration
(a) Perfect crystal without
vacancies
(c) An atom in the bulk diffuses
to fill the vacancy thereby
displacing the vacancy towards
the bulk.
(b) An energetic atom at the surface breaks
bonds and jumps on to a new adjoining position
on the surface. This leaves behind a vacancy.
(d) Atomic diffusions cause the vacancy to
diffuse into the bulk.
Fig. 1.43: Generation of a vacancy by the diffusion of an atom to
the surface and the subsequent diffusion of the vacancy into the
bulk.
From Principles of Electronic Materials and Devices, Second Edition, S.O. Kasap (© McGraw-Hill, 2002)
http://Materials.Usask.Ca
(a) A vacancy in the crystal.
(b) A substitutional impurity in the crystal. The
impurity atom is larger than the host atom.
(c) A substitutional impurity in
the crystal. The impurity atom
is smaller than the host atom.
(d) An interstitial impurity in the crystal. It
occupies an empty space between host atoms.
Fig. 1.44: Point defects in the crystal structure. The regions around
the point defect become distorted; the lattice becomes strained.
- Schottky defect (cation-anion pair missing) & Frenkel defect (host into interstitial position)
Schottky defect
Frenkel defect
(a) Schottky and Frenkel defects in an ionic crystal.
Substitutional impurity.
Doubly charged
(b) Two possible imperfections caused by ionized
substitutional impurity atoms in an ionic crystal.
Fig. 1.45: Point defects in ionic crystals
1.8.2 Line Defects: Edge and screw dislocations
Edge dislocation line
(a) Dislocation is a line defect. The dislocation shown runs into the paper.
Compression
Tension
(b) Around the dislocation there is a strain field as the atomic bonds have
been compressed above and stretched below the islocation line
Fig. 1.46: Dislocation in a crystal is a line defect which is accompanied
by lattice distortion and hence a lattice strain around it.
From Principles of Electronic Materials and Devices, Second Edition, S.O. Kasap (© McGraw-Hill, 2002)
http://Materials.Usask.Ca
A
C
D
Dislocation line
(a) A screw dislocation in a crystal.
B
Dislocation A
line
Atoms in the
upper portion.
Atoms in the
lower portion.
D
C
(b) The screw dislocation in (a) as viewed from above.
Fig. 1.47: A screw dislocation involves shearing one portion of a
perfect crystal with respect to another portion on one side of a line
(AB).
New molecule
Growth spiral on the surface of a
Fig. 1.49: Screw dislocation aids
polypropylene crystal due to screw
crystal growth because the newly
dislocation aided crystal growth.
arriving atom can attach to two or
(SOURCE: Photo by Phillip Geil, Courtesy of
three atoms instead of one atom and Case Western Reserve University.)
thereby form more bonds.
From Principles of Electronic Materials and Devices, Second Edition, S.O. Kasap (© McGraw-Hill, 2002)
http://Materials.Usask.Ca
1.8.3 Planar Defects: Grain boundaries
Nuclei
Crystallite
Liquid
(a)
(b)
Grain
Grain boundary
(c)
Fig. 1.50: Solidification of a polycrystalline solid from the melt. (a)
Nucleation. (b) Growth. (c) The solidified polycrystalline solid. For
simplicity, cubes represent atoms.
From Principles of Electronic Materials and Devices, Second Edition, S.O. Kasap (© McGraw-Hill, 2002)
http://Materials.Usask.Ca
Foreign impurity
Self-interstitial type atom
Void, vacancy
Strained bond
Grain boundary
Broken bond (dangling
bond)
Fig. 1.51: The grain boundaries have broken bonds, voids, vacancies,
strained bonds and "interstitial" type atoms. The structure of the grain
boundary is disordered and the atoms in the grain boundaries have higher
energies than those within the grains.
From Principles of Electronic Materials and Devices, Second Edition, S.O. Kasap (© McGraw-Hill, 2002)
http://Materials.Usask.Ca
1.8.4 Crystal Surfaces and Surface Properties
H2O
Dangling bond
Surface
Reconstructed
surface
Absorbed
Oxygen
O
H
H2
Surface atoms
Bulk crystal
Fig. 1.52: At the surface of a hypothetical two dimensional crystal, the
atoms cannot fulfill their bonding requirements and therefore have
broken, or dangling, bonds. Some of the surface atoms bond with each
other; the surface becomes reconstructed. The surface can have
physisorbed and chemisorbed atoms.
From Principles of Electronic Materials and Devices, Second Edition, S.O. Kasap (© McGraw-Hill, 2002)
http://Materials.Usask.Ca
1.10 Glasses and Amorphous Solids
Silicon (or Arsenic) atom
Oxygen (or Selenium) atom
(a) A crystalline solid reminiscent to
crystalline SiO2.(Density = 2.6 g cm-3)
(b) An amorphous solid reminiscent
to vitreous silica (SiO2) cooled from
the melt (Density = 2.2 g cm-3 )
Fig. 1.56: Crystalline and amorphous structures illustrated
schematically in two dimensions.
From Principles of Electronic Materials and Devices, Second Edition, S.O. Kasap (© McGraw-Hill, 2002)
http://Materials.Usask.Ca
Dangling
bond
(a) Two dimensional schematic
representation of a silicon crystal
H
H
H
H
(b) Two dimensional schematic representation
of the structure of amorphous silicon. The
structure has voids and dangling bonds and
there is no long range order.
(c) Two dimensional schematic representation
of the structure of hydrogenated amorphous
silicon. The number of hydrogen atoms shown
is exaggerated.
H
H
Fig. 1.58: Silicon can be grown as a semiconductor crystal or as an
amorphous semiconductor film. Each line represents an electron in a
bond. A full covalent bond has two lines and a broken bond has one line.
From Principles of Electronic Materials and Devices, Second Edition, S.O. Kasap (© McGraw-Hill, 2002)
http://Materials.Usask.Ca
[Homework]
1. Prob. #1.1
2. Prob. #1.18