Nuclear Physics Questions & Answers: Summary 18.1

Summary questions for 18.1
Question 120S: Short Answer
1.
Mass of alpha particle
Energy 1 MeV
1 .
6 10
19 10
6
4 10 3
N
A
7 10
27 kg kg .
J
1 .
6 10 13
Therefore
1
2 mv
2 1 .
6 10
13
J
J .
4
6 .
10
02
3 kg
10 23 v
2 1 .
6 10 13
7 10
27 kg
J
7 10
6 m s
1
.
2. The total number of ionising events is
5 .
3 10
6 eV
30 eV
1 .
8 10
5
The average number of ionisations per mm is
1 .
8 10
5
4 .
7 10 3 mm –1
38 mm
As the alpha particle reaches the end of its range it slows down. This means that it is close to an air molecule for a longer time, so increasing the chance of ionisation.
3. 0.142 MeV = 0.142 × 10
6
×1.6 ×10
–19
J eV
–1
= 2.27 ×10
–14
J
E
hf f
0 .
142 1 .
6 10
13
6 .
63 10
34 J Hz
1
J c
3
λ f
.
42 10
19
Hz .
λ
3.00
10
8 m s
–1
3.42
10
19
Hz
8 .
77 10 12 m
4. The activity must not exceed 10 000 Bq per litre.
0 .
693 t
1 / 2
0 .
693
1 .
27 10
5 s
5 .
457 10 6 s 1 .
1 Advancing Physics
activity λ N
10000 Bq l –1
N
5.457
10
– 6 s
–1
N
1 .
83 10
9 atoms
The maximum number of moles of bromine atoms dissolved in 1 litre of the stream water is
1 .
83 10 9 atoms l –1
6 .
02 10 23 atoms mol –1
3 .
04 10
–15 mol l
–1
5. t
1 / 2
87 year 365 day year
–1 24 hour day
–1 60 min hour
–1 60 s min
–1
2 .
74 10 9 s .
0 .
693
t
1 / 2
0 .
693
2 .
74 10
9 s
2 .
52 10 10 s 1 .
The activity, A , is given by
N . To find N
0
, use N
A
= 6.02 10
23
mol
–1
, then
6.
N
0
2000 g
2 38 g mol
–1
6 .
02 10
23 mol
The initial activity, A
0
, is given by
N
0
:
N
0
2 .
52 10
10 s
1 5 .
06 10
24
1 .
3 10 15 Bq .
–1
A ln
/
0 .
A
0
1
e
t
t
2 .
52 10 10 s 1 t
5 .
06 10
24 t
9 .
13 10
9 s
289 years .
Alternative method:
( 1
2
) n 0 .
1 n t
3 .
32
3 .
32 87
289 years years
.
Like any other alpha emitter, plutonium will be dangerous if ingested so that the alpha particles are emitted inside the body.
2 Advancing Physics
N / e
N
0
t t
1 / 2
0 .
54
0 .
54
5730 years
ln 2 / t
1 / 2
0 .
693
5730 years ln 0 .
54
1 .
209 10
4
6 .
162
( year )
1
1 .
209 10 4 ( year ) 1 t t
5100 years .
Note: The
14
C date calculated above is earlier than the archaeologists’ estimate; there is much evidence that the Iceman lived about 4000 years ago. Radiocarbon dating is subject to uncertainties and the calculations are more complex than outlined above.
Change in energy:
Change in mass
Question 200S: Short Answer
1.
Mass of
Mass of
H plus Li two He
1.0073
u
2
8.0233
u.
4.0015
u
7.0160
u
Difference m
8 .
0030
8.0030
u u .
8 .
0233 u
0 .
0203 u .
So we can find the mass difference in kg:
m
0 .
0203 u 1 .
6605 10
27 kg
3 .
3708 10
29 kg .
2. Increase in energy:
E
2 8 .
5 MeV 0 .
8 MeV
16 .
2
In joules:
MeV .
E
( 16 .
2 10 6 eV ) ( 1 .
6 10 19
2 .
6 10
12
J .
J eV 1 )
3.
E
m
2 .
60 10
12
3 .
37 10
29
J kg
7 .
7 10
16
J kg
1
.
3 Advancing Physics
4. If
E = mc
2
, then c
2
= 7.7 10
16
J kg
–1
, so c = 2.8 10
8
m s
–1
.
5. The force on a moving charged particle is q v B . If the charge q changes sign, the direction of the force is reversed, so the curvature is opposite.
6. The mass of an electron or positron is equal to:
( 5 .
5 10 4 u ) ( 1 .
66 10 27 kg ) 9 .
1 10 31 kg .
From E rest = m c
2
, the rest energy of an electron–positron pair is:
E rest
2 9 .
1 10 31 kg ( 3 10 8 m s 1 ) 2
1 .
6 10
13
J .
If this energy is supplied by a photon of energy E = h f , then: f
1 .
6
6 .
63
10
13
10
34
J
J
Hz
1
2 .
5 10 20 Hz .
This is the frequency of a gamma ray.
7. The mass difference is:
2 .
0136 u ( 1 .
0073 u 1 .
0087 u )
In kg the mass difference is:
0 .
0024 u ( 1 .
66 10
27 kg ) 3
0
.
98
.
0024
u
10
30
.
kg .
8.
Binding energy 3 .
98 10
30
– 3 .
58 10
13 kg
J
( 3 10
8 m s
1
)
2
–
3 .
58
1 .
6
10
13
10
19
J
J eV
1
– 2 .
2 MeV .
– 2 .
2 10
6 eV
9. The deuteron has two nucleons so the binding energy per nucleon is –2.2 MeV / 2 = –1.1 MeV.
10. As a percentage the mass difference is equal to:
1 .
0
0073
.
0024 u 1 .
u
0087 u
1 .
2 10
3 100
0 .
1 % ( approximat ely ).
11.
Mass before
Mass after
235.0439
u
132.9152
u
1.0087
98.9116
u u 236.0526
u.
( 4 1.0087
u)
Mass
235 .
8616 difference u .
236.0526
0 .
191 u .
u 235 .
8616 u
Change in rest energy
0.191
u ( 1.66
10
-27 kg
1 .
6 10
19
)
J
( 3 eV
10
8
1
1 .
78 10
8
178 MeV .
eV m s
1
)
2
The ratio is given by:
4 Advancing Physics
m / m
0 .
191 u / 236 u
8 .
1 10
4
~ 0 .
1 %.
Summary questions for 18.2
Question 210S: Short Answer
1. Radium-226 can be produced as part of the uranium-238 decay chain. Uranium-238 has a half-life of 4.51 10
9
years. Radium-226 is continually being produced in minerals containing uranium.
2. Thorium. The complete decay chain involves the loss of seven alpha particles (
4
2
) and four beta
). This represents a loss of 7 4, i.e. 28, in mass number and (7 2 – 4), i.e. 10, in atomic number. X is therefore an isotope with mass number (235 – 28), i.e. 207, and atomic number (92 – 10), i.e. 82. This is lead,
208
82
Pb
.
3.
235
92
U 1
0 n 137
55
Cs 94
37
Rb 3 1
0 n .
The reaction produces more neutrons than it absorbs, this will cause a ‘chain reaction’.
To see why the products of the reaction are likely to be radioactive you need to consult the plot of neutron number against atomic number for the known stable nuclei. For elements with atomic numbers up to about 30 the number of neutrons in the nucleus is the same as the number of protons if the nucleus is stable. For higher atomic numbers the ratio of neutrons to protons gradually increases to 1.5. Look at the position of both
137
55
Cs
on the plot and you will see that they both have a considerable excess of neutrons. They are therefore likely to be radioactive. To become more stable the nuclides need to decrease the neutron to proton ratio.
The emission of a beta particle does this, increasing the number of protons by one and decreasing the number of neutrons by one. These isotopes are therefore likely to decay by emitting beta particles.
4. When boron captures a neutron it is transformed into a stable isotope. If the control rods are pushed into the reactor more neutrons are absorbed, causing the chain reaction to slow down. If they are pulled out the chain reaction will proceed more vigorously.
5.
27
13
Al
30
15
P
4
2
30
14
Si
30
15
P
0
1 e
1
0 n
6.
131
53
I
131
54
Xe
0
1 e beta decay
67
31
Ga
11
6
C
11
5
0
1 e
B
99 m
43
Tc 99
43
Tc
67
30
0
1 e
Zn positron nuclear electron capture emission rearrangem ent
7.
239
94
Pu
239
94
Pu
2
1
0 n 241
95
Am
4
2
242
96
Cm
0
1 e
1
0 n
5 Advancing Physics
242
96
Cm 4
2
245
98
Cf 1
0 n
252
98
Cf 9
5
B 257
103
Lw 4
1
0 n.
Americium is commonly found in homes where it is used in smoke detectors.
8.
244
94
Pu 48
20
Ca 289
114 new element 3 1
0 n
Fission and fusion – practice questions
Question 250S: Short Answer
1.
235
92
U 1
0 n 236
92
U 90
36
Kr 144
56
Ba 2
1
0 n kinetic energy
2. m = 235.043923 u – 1.008665 u = 236.052 588 u
3. m = 89.919528 u + 143.922941 u + 1.008665 u + 1.008665 u =235.859 799 u
4. m =236.052 588 u – 235.859799 u = 0.192 789 u; energy lost
5.
E = 0.192 789 u × 931.3 MeV u
–1
=179.49 MeV
6. m = 137.905241 u + 95.934284 u + 1.008665 u + 1.008665 u =235.856855 u
m = 236.052 588 u – 235.856855 u = 0.195733 u
E = 0.195733 u × 931.3 MeV u
–1
= 182.2 MeV
7.
2
1
H 2
1
H 3
2
He 1
0 n
8. mass of two hydrogen-2 = 2 × 2.014102 u = 4.028204 u mass of helium-3 plus neutron = 3.016030 u + 1.008665 u = 4.024695 u
m = 4.028204 u – 4.024695 u = 0.003509 u
E = 0.003509 u × 931.3 MeV u
–1
= 3.27 MeV
9. mass of hydrogen-2 plus neutron = 2.014102 u + 1.008665 u = 3.022767
m =3.022767 – 3.016050 u = 0.006 717 u.
10. The reaction can occur with a release
E = 0.006 717 u × 931.3 MeV u
–1
= 6.26 MeV
Fusion in a kettle?
Question 260S: Short Answer
1.
2
1
H 2
1
H 3
2
He 1
0 n
2. m = (3.016 030 u + 1.008 665 u) – 2 × 2.014 102 u = – 0.0035 u
3. 0.003509 u × 931 × 10
6 eV u
–1
× 1.6 × 10
–19
J eV
–1
= 5.23 10
–13
J
4. 18 g
5. 1 litre of water has a mass of 1 kg.
6 Advancing Physics
number of moles = 1000 g / 18 g mol
–1
~ 56 mol
6. 56 mol × 6.02 × 10
23
mol
–1
= 3 10
25
7. (3.4 ×10
25
)/7000= 4.9 10
21
8. energy released = 4.9 ×10
21
× (5.23 ×10
–13
J) = 2.49 10
9
J
9. (2.49 ×10
9
J)/(4200 J kg
–1
K
–1
× 100 K) = 6000 kg = 6000 litres
Fission in a nuclear reactor – how the mass changes
Question 270S: Short Answer
1. Energy = (20 10
12
W h yr -1 × 3600 J W h
–1
)/(3.16 10
7
s yr -1 ) = 5.7 10
9
J s
–1
.
2. Number of fissions per second = (5.7 10 9 J s fissions s -1 .
–1
)/(200 10
6
eV × 1.6 10
–19
J eV
–1
) = 1.8 10
20
3. Mass of U-235 fissioned per second = 1.8 10
20
s
–1
× 235.04394 u × 1.66 × 10
–27
kg u
–1
= 7.0 ×
10
–5
kg s
–1
= 70 mg s
–1
.
4. Mass change per second = (5.7 10
9
J s
–1
) / ((3 10
8
m s
–1
)
2
) = 6.3 × 10 -8 kg s
–1
= 60 g s
–1
5. Disintegrations per second = ((1 10
19
J s
–1
)/(200 10
6
MeV × 1.6 10
–19
J eV
–1
)) × (100/40) =
7.8 10
19
s
–1
6. Mass per second = 7.8 10
26
s
–1
× 235.04394 u × 1.66 × 10
–27
kg u
–1
= 3.0 × 10
–5
kg s
–1
7. Mass = 3.0 × 10
–5
kg s
–1
× years × 3.2 × 10
7
s y
–1
= 2900 kg
8. Mass = 2800 kg ×(100/0.7) = 400 000 kg
9. Lower limit.
Radon:
Healthy or harmful?
Question 40C: Comprehension
1.
226
88
Ra 222
86
Rn 4
2
He 218
84
Po 4
2
He
There is an OHT in this chapter which provides the full scheme.
2. The radon was not entering his lungs; radon was decaying in his mouth (and stomach, intestines etc), and it was here that the damage was done. The alpha decay would not penetrate as far as his lungs.
3. Look around health food shops and you will find similar claims made for a variety of products –
Ginkgo biloba , Aloe vera , Chinese herbs etc. In other parts of the world, such claims are often
7 Advancing Physics
made for products extracted from obscure parts of rare and dangerous animals – tiger bones etc.
4. At Badgastein, the level is 170 Bq l
–1
, or 170 000 Bq m
–3
. This is almost 1000 times the level at which buildings are regarded as dangerous.
5. Typical annual exposure is 20 Bq m
–3
for 24 365 hours, or 175 200 Bq h m
–3
. Exposure during
5 hours at Badgastein would be 170 000 Bq m
–3 5 = 850 000 Bq h m
–3
. In fact, just 1 hour in the healing galleries would give you an exposure similar to the typical annual exposure in the UK.
6. This approach is similar to homeopathy, where minute doses of otherwise harmful substances are said to stimulate the body’s defence systems. It also resembles inoculation against infectious diseases, in which a weak infection is used to build up the body’s immune system so that antibodies to the disease are produced.
7. According to the Spa publicity, radon breathed in during treatment in the healing galleries has
‘completely broken down’ within 3 hours. This is much shorter than the radioactive half-life, which suggests that the biological lifetime is shorter than the half-life.
How safe are x-rays?
Question 50C: Comprehension
1. In the earlier technique the contrast was directly dependent on the relative densities of the material. The fluorographic technique produces more contrast as it depends on a cube factor of the atomic number.
2. The soft x-rays are absorbed by the glass screen on the front of the television and do not reach the soft tissue. However, do not view the screen from too close a distance.
3. The filter removes the ‘soft’ x-rays (x-rays of long wavelength).
4. Long wavelengths are associated with lower energy levels, shorter wavelengths with high photon energies. The expression given tells us that a small increase in the energy of the x-rays ( E ) greatly decreases the absorption of the energy – note the cube term. The atomic number ( Z ) of minerals such as calcium ( Z = 20) contained in bone is relatively higher than that of soft tissue which is mainly carbon based. Since the absorption of x-rays by the tissue depends on the cube of Z , the bone must absorb more radiation. You could do a rough calculation based on Z numbers but you will not obtain the estimate of nearly six times unless you had the exact composition of the tissues.
5. E = hf = h ( c / ) = 6.63 × 10
–34
J s × ((3.00 × 10
8
m s
–1
)/(1.76 × 10
–11
m)) = 1.13 10
–14
J
6. (1.76 ×10
–11
m)/(3 ×10
–13
m) = 59
The energy of the shorter x-rays will be about 60 times greater causing more damage to the cells
7. The shadowgram used a lower energy beam with longer wavelengths over a wider area of the body. Therefore the body would absorb a great deal of ionising radiation. Fluorography used a higher wavelength beam, which is not absorbed so easily, over a smaller area of the body.
However, the beam remained on for a long time. The table suggests that the received dose of ionising radiation is far less for the shadowgram.
8. Currently the absorbed dose is measured in gray (Gy) which measures the energy absorbed by the tissue in J kg
–1
. The roentgen does not measure the actual absorption by the tissue.
8 Advancing Physics
The mass x-ray programme
Question 60C: Comprehension
1. Most of the harmful effects of x-ray exposure, e.g. cancer, did not appear until after a delay of over 20 years.
2. The only way of preventing the disease spreading was early diagnosis and isolating the infected people from the rest of the community. X-rays were the only way to detect the disease in its early stages, because the symptoms mimicked those of bronchitis.
3. Lead has a high atomic number and will absorb most of the x-ray photons.
4. Improvements include: lead shutters control the size of the beam and therefore the area irradiated; intensifying screens improve the contrast so an image can be obtained from a much lower level of radiation; exposure time is now down to 1 / 100 s; limiting the number of x-ray exposures for any one patient (there are no recommended limits); better maintenance of equipment.
5. The benefits include: better diagnosis and consequentially more specific treatment; with appropriate treatment, it follows that the lifespan may be increased and/or lifestyle improved; less wastage on inappropriate treatment, lengthy hospitalisation and nursing care; the patient may be able to return to work.
Set this against the ionising effect of a prolonged exposure to a particular organ or tissue with subsequent cancers appearing in later life. Also there is a chance of hereditary damage if the patient goes on to have children. The original price of the equipment is high. The cost of maintenance, training of radiographers and technicians, salaries, high-voltage electric power supply all feature in medical decisions today.
Some of the dangers of flying too high
Question 70C: Comprehension
1. From about 3 to 5 mSv; yes for female crew members for whom the recommended maximum is 1 mSv per annum.
2. Only gamma radiation is likely in aircraft; data about the effects of proton and neutron irradiation would be more helpful.
3. Frequent flyers – especially couriers.
4. Twenty-four hours to Thailand and back, at a maximum say of 8 Sv h
–1
, results in an equivalent dose of 200 Sv.
Two weeks in Cornwall provides an equivalent dose of 8000 Sv year
–1
/ 26 = 300 Sv. (It’s usually warmer in Thailand – but it costs more.)
5. Alpha particles are easily stopped, e.g. by paper and clothing. Protons are likely to be much more penetrating and cause ionisation inside tissue. It is this that provides the risk of genetic and tissue damage.
6. Ignoring relativistic effects! Use E = ½ m v
2
to give:
9 Advancing Physics
v
2
( 2 10
10 eV) ( 1 .
1 .
7 10
27
6 10
19 kg
1 .
9 10
18
( m s
1
)
2
J) v
1 .
4 10 9 m s
1 which is greater than the speed of light. This is impossible – the simple formula doesn’t work. The energy is about 20 times the rest energy, with relativistic factor being equal to the ratio total energy / rest energy. The true speed can be found from:
20
1 or v
0 .
999 c
1 v
2 c
2
Hot news:
A radioactive leak in Japan
Question 80C: Comprehension
1. The becquerel is the SI unit of activity of a radioactive material. 1 Bq of material gives one radioactive decay per second. It’s not strictly correct to talk about becquerels of radiation; the activity of the material is measured in Bq, but the radiation would be measured in other units, such as the amount of energy released per second.
2. 46 000 Bq / 20 000 kg = 2.3 Bq kg
–1
.
3. You decide.
4. Terms like ‘flooded’ make the story more dramatic. The fact that cleanup workers were only allowed in for 3 hours each day also adds to the sense of alarm.
5. Details such as the length of the fissure and the number of becquerels released add to the authenticity.
6. Like most of the materials in our body, potassium-40 comes from our food.
7. Assuming a mass of 70 kg (approx. 11 stone), and that 4166 Bq represents most of the radioactive material in our bodies, we have a concentration of 4166 Bq / 70 kg = 60 Bq kg
–1 approximately, a lot higher than the concentration in the water which leaked at Tsuruga.
8. You may feel that the leak was hardly worth reporting. Alternatively, you may feel that there may have been a serious leak but that it was incorrectly reported.
Industrial radiography
Question 90C: Comprehension
10 Advancing Physics
1. A thin-walled pipe needs low-energy gamma rays, so choose thulium-170 (or ytterbium-169).
Thick casting needs high-energy gamma rays to penetrate it, so choose cobalt-60.
2. The most energetic photons would pass through a thin object with scarcely any absorption. It would be very difficult to see any difference between thick and thin areas.
3. Alphas would be completely absorbed, even by a thin sheet. Gamma rays would only be very slightly absorbed.
4. A gamma source emits radiation at all times, and requires careful storage. An x-ray machine (or other accelerator) emits no radiation when it is switched off, so storage poses no hazard.
The smoke detector
Question 100C: Comprehension
1. Americium appears beyond uranium ( Z = 92) in the periodic table.
2. Alpha radiation is more strongly ionising than beta or gamma radiation. This means that its range in air is shorter, as it is more easily absorbed. (The range of alpha particles is typically a few centimetres.) A small amount of smoke will be sufficient to absorb the alpha radiation.
3. If an alpha source enters the body, its radiation can cause cancer. It is thus essential to ensure that the americium source from a smoke detector does not enter the environment. They should not be dumped at a refuse tip, for example, where the material might enter the water supply. The container must not be broken, or the americium may blow away, later to be breathed in.
4. In both, alpha radiation ionises air and thus allows a current to flow.
5. Because carbon dioxide is denser than air, the range of alpha radiation is less in CO
2
than in air.
Position the alpha source at a distance of, say, 5 cm from the detector. In air the radiation would just reach the detector; in CO
2
it wouldn’t, and the alarm would sound.
Radioisotope tracers in the oil industry
Question 110C: Comprehension
1.
Isotope
Hydrogen-1
Deuterium
Tritium
Symbol
1
1
H
2
1
H
3
1
H
1
1
Number of protons
1
1
2
Number of neutrons
0
1
1
Number of electrons
1
11 Advancing Physics
2. Helium is formed – in this case, helium-3, a very rare isotope.
3
1
H 3
2
He
0
1
β
(The symbol represents an antineutrino, which is always emitted during –
decay.)
3. It would be very difficult to detect alpha radiation, since it is absorbed by very small thicknesses of materials. Also, it is undesirable to introduce an alpha emitter into the environment in an uncontrolled way, since if such sources are ingested, they are extremely hazardous.
4. If it took a long time for the radioisotope to travel from one arm to the other, via the heart, this would indicate the presence of heart disease; the veins and arteries must be partially blocked, slowing the flow of blood.
Radiation protection and dosimetry
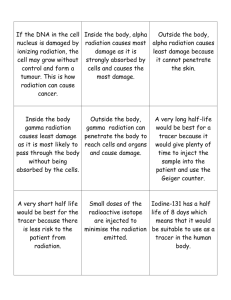
Question 130C: Comprehension
1. In the first plant there is a danger of breathing in particles of the materials being processed – the main danger being from ingested alpha emitters. In the second the design of the plant cuts down the radiation via at least the second two of the ways mentioned in the passage.
2. In the power plant there is a possibility of danger from both beta and gamma radiation, and accidents might produce ‘leaks’; in a hospital the radiation is not only less penetrating, but much more easily controlled (e.g. by switching off the x-ray machine).
3. A good answer would refer to the thicknesses of absorber and to the distances from the sources.
An office worker would need a film badge as there is likely to be above background radiation throughout the plant.
4. A significant fraction of the beta radiation is absorbed by the plastic, so resulting in different exposures to the film. Plastics are poor absorbers of gamma radiation, metals are more dense and so would affect the gamma more.
5. Alpha particles are highly ionising, so penetrate very little, not reaching the film on the badge.
6. If there is an accident or a worker makes a mistake, the level and intensity of the radiation is too high to be recorded accurately by a film badge: it would be hard to distinguish between a large dose and an even larger one.
7. Silver atoms in the emulsion have electrons knocked out of them and become sites at which silver metal forms when the film is developed.
Radiation processing
Question 160C: Comprehension
1. Use the formula
I x
I
0 e
x with the given values:
1 10
8 e
77 x
12 Advancing Physics
which rearranged gives
10 8 e
77 x which is better written as
10
8 e
77 x
Take logs to base e: so for lead x
18
77
.
42 m
–1 for concrete
x
18 .
42
1 8 m –1 for water
0 .
24 m
1 .
02 m x
18 .
42
7 m
–1
2 .
6 m
2. 300 kCi means there are 3 10
5 3.7 10
10
events (emissions) per second, i.e. 11.1 10
15 events.
Each event provides 3 MeV of energy and most is absorbed in the lead, heating it. 1 eV is equivalent to 1.6 10
–19
J, so total energy dissipated per second can be found as follows: energy per second 11.1
10
15 s
–1 3 10
6 eV 1.6
10
-19
J eV
–1
5330 J s –1
The specific thermal capacity ( c ) of lead is 129 J kg
–1
K
–1
. If the mass ( m ) of lead used is 3 tonnes (3 10
3
kg) we can use the thermal transfer formula
E = mc
T to estimate the rise in temperature per second produced:
E
T
mc
T
E
mc
5330 J s
–1
3000 kg 129 J
–1 kg
–1
K
–1
0 .
014 K s
–1
50 K h
–1
This is a lot! So a cooling system is vital.
3. A good answer would refer to time spent near the source, shielding and separation from the source.
Teletherapy:
Treatment of tumours by ionising radiation
Question 180C: Comprehension
1. Beta radiation is used. Alpha radiation is more ionising and might be a sensible answer, but it has too short a range – it cannot penetrate deeply enough into tumours. Gamma rays have too long a
13 Advancing Physics
range and would pass too easily through tissues, so would not provide a localised effect.
2. Gamma rays must be used. Alpha radiation would not even reach the body, being absorbed by the air. Beta is likely to be absorbed by the skin and is unable to reach any deep-seated tumours.
3. The total dose is found by adding up all the individual doses. So we get:
2 Gy per day 5 days per week 6 weeks 60 Gy .
Since 1 Gy = 1 J kg
–1
, 60 Gy in 0.05 kg (50 g) will produce an energy dissipation of 3 J.
4. energy per disintegra tion 2.8
10 6 eV 1.6
10 -19 J eV -1
4 .
48 10
13
J number of disintegra tions per second 3 10
13 s
–1 power 4 .
48
13 .
4 W
10 –13 J 3 10 13 s –1
So 3 10
13
disintegrations per second produce 13 W of power.
5. 10.5 years is two half-lives so the activity will have halved twice. It will now be 7.5 10
12
Bq. The use of the machine is irrelevant – the cobalt continues to decay regardless of its surroundings.
When the machine is used, the radiation is allowed to escape, rather than being absorbed by shields.
6. The curve you are given shows the energy deposited at a given depth, so it shows the rate of loss of energy from the beam. Hence this curve is related to the slope of the graph of intensity against depth. The most energy is deposited at the surface of the body – the skin. So even when care is taken to expose the tumour from a number of angles the patient’s skin will still be exposed to a large dose of radiation which can cause burns. In more modern machines, which use higher energies, the energy deposited at the skin is quite low, and the peak can be deeper down so the skin is relatively unaffected, leading to much more comfortable treatment.
The peak of absorption of the 22 MeV beam is just about 6 cm beneath the skin. This can be moved by passing the beam through an equivalent of 4 cm of body tissue (a tissue ‘phantom’ such as water would be adequate) before it reaches the patient. Alternatively the equivalent thickness of a more absorbing material would be more easily introduced – a thin foil of copper for example. Atoms with a higher atomic number more readily absorb the radiation.
7. The graph shows the shape of the slope of the intensity so the actual intensity must vary something like this:
14 Advancing Physics
10000
8000
Intensity of 22 MeV x-ray in the body
Small slope initially where absorption is low
6000
4000 Slope is greatest where dose is greatest - around 6 cm
2000
Small slope where absorption decreases again
0
0 5 10
Depth / cm
15 20
Using gamma rays
Question 190C: Comprehension
1.
76
33
As 1
0 n 77
33
As
2.
65
28
Ni
max 2130 keV
65
Cu
29
1481 keV
366 keV
1115 keV
1481 keV 1115 keV
0
3. One of the nuclides into which the original nucleus splits
4. Half life is 30 years, more than half still present now.
5. Beta particles can be absorbed by the sample itself
15 Advancing Physics
6. Products of irradiation by neutrons
Life and death of a nuclear reactor
Question 220C: Comprehension
1. The offices and the electrical gear have only background levels of radioactivity associated with them. They can be dismantled and removed relatively quickly. The reactor itself is highly radioactive and also contains toxic materials. It needs to be treated differently so that human operators are not put at risk from the materials in it.
2. Simply encase the whole reactor and leave it there. This would create an eyesore and might not ultimately be safe if the encasing materials broke down with time. Alternatively, use remote-controlled machines to dismantle the reactor from a distance. There are then the problems of transportation and/or storage of the active materials and the danger of release into the environment.
3. The unstable nucleus is rich in neutrons, so the neutron is likely to be converted to a proton, with the emission of an electron to conserve charge. The nucleus is likely to be a beta ( -) emitter
Nuclide
Tritium (hydrogen-3)
Carbon-14
Calcium-41
Iron-55
Nickel-63
Number of half-lives in
135 years
Almost 11
0.02
0.00135
50
About 1
Activity as a fraction of the original
1/(2
11
) = 5 ×10
–4
Virtually unchanged
Virtually unchanged
1/(2
50
) 10
–15
1/2 = 0.5
5. The table below shows the final predicted radioactivity concentrations:
Material Mass / tonne Activity after 135 years /
Bq
Radioactivity concentration
/ Bq kg
–1
Steel pressure vessel
Graphite
Carbon steel
Stainless steel
Concrete
16 Advancing Physics
1900
2800
140
21 000
4 10
13
2 10
14
3 10
14
8 10
11
2.1 10
7
7.14 10
7
2.14 10
9
3.81 10
4
Material Mass / tonne Activity after 135 years /
Bq
Concrete pressure vessel
Graphite 3100
Carbon steel
Stainless steel
Concrete
11 000
170
38 000
2 10
13
5 10
12
2 10
13
1 10
10
Radioactivity concentration
/ Bq kg
–1
6.45 10
6
4.55 10
5
1.18 10
8
2.63 10
2
6. Only the stainless steel would be well outside the limits (about 100 or 1000 times larger than low-level). Everything else is either well below or (in the case of graphite and carbon steel from the steel pressure vessel reactors) just above.
Power in space
Question 240C: Comprehension
1. The plutonium gradually decays.
2.
Efficiency electrical thermal power
(heat) out power in
0 .
067
6 .
7 %.
296 W
4394 W
3.
Decay probabilit y ln 2 / 87 .
7 years
7 .
9 10
3 year
The output after 11 years is therefore
296 W e
t 271 W .
1 .
In fact, the anticipated power output is less than this (210 W). As the plutonium cools, the RTG becomes even less efficient.
4. Ceramic materials are hard, stiff, strong and brittle. They have high melting points and are chemically unreactive. The fuel is thus more likely to remain intact during a severe impact. If it comes into contact with other materials, e.g. water, it will not corrode.
5. RTGs could be useful in deep-sea exploration; they could also provide power for long-term applications in remote places, e.g. for environmental monitoring instruments in polar regions.
6. Step 1: Start by calculating the number of Pu nuclei which must decay each second to provide the thermal output quoted, given the energy released by each decaying nucleus. This is the activity A . The following data are needed: energy released per decay = 5.5 MeV = 5.5 (1.6 10
–13
J MeV
–1
) = 8.8 10
–13
J thermal power output = 4394 W.
17 Advancing Physics
So
A
4394 W / 8 .
8 10
13
5 .
0 10 15 s 1 ( or Bq ).
J
Step 2: Next we use
A
N to calculate the number N of undecayed Pu nuclei. (Find the value of in s following data: half-life T
1/2
= 87.7 years = 2.76 10
9
s decay probability = ln 2 / T
1/2
= 2.51 10
–10
s
–1
.
The number of undecayed nuclei is therefore
N
A /
–1
.) We need the
5 .
0 10 15
2 .
51 10
10 s 1 s
1
2 .
0 10
25
.
Step 3: Finally, use the information that 1 mole of Pu-238 atoms has a mass of 238 g, approximately, and contains N
A
atoms (i.e. mass of N
A
atoms = 238 g). Therefore mass of 2 10 25 atoms
238 g mol –1 2 10 25
6 .
02 10 23 mol –1
7878 g
7 .
9 kg .
The cost of taking a chance
Question 10D: Data Handling
Here are examples of each type; you may have found alternative examples:
1. Crossing the road.
2. Medical radiation exposure.
3. Background radiation exposure.
4. Exposure of aircrew to cosmic rays.
5. Radon exposure of residents of Cornwall.
6. Freefall parachuting.
7. Lifetime risk of dying in road accident = 75 in 15 000 = 1 in 200.
8. If you have a circle of friends and acquaintances which is a few hundred strong, there is a very good chance that one of them will die in a road accident.
9. The smoker’s lifetime risk is much higher because the data relate to the entire UK population.
Only a fraction are smokers, so their risk is proportionately higher. In fact, about 1 in 4 of heavy smokers dies of a smoking-related disease.
10. Total cost / number of cancers avoided = £1750 100 000 / 7500 = £20 000. This seems a relatively small price to pay to save a life. However, in practice it is up to individuals in affected
18 Advancing Physics
areas to pay for the necessary safety measures, and the cost often seems large for a risk which may seem rather small.
adioactive decay series
Question 140D: Data Handling
1. Graph: The advantage is that the decay path is very clear and the difference between alpha and beta decay can be seen at a glance. The disadvantage is that it is difficult to include all the relevant data such as half-lives without it becoming crowded.
Table: The advantage is that more data such as nucleon numbers can be added without overcrowding. The disadvantage is that the chain is not so obvious, and it is easy for errors to creep in.
Chain: The advantage is that the series is obvious. The disadvantage is that it becomes crowded if other information is added, and it needs a certain amount of interpretation to read.
2.
232
90
Th 228
88
Ra 228
89
Ac 228
90
Th 224
88
Ra
220
86
Rn 216
84
Po 212
82 and for the second series:
232
90
Th 228
88
Ra 228
89
Pb
Ac
212
83
Bi 212
84
228
90
Th
Po
224
88
Ra
208
82
220
86
Rn 216
84
Po 212
82
Pb 212
83
Pb
Bi 208
81
Tl 208
82
Pb .
3. In each case the final product is the stable nuclide lead-208.
4. The two alpha decays cause a reduction in the mass number by 8 and the atomic number by 4.
The beta decay has no effect on the mass number but increases the atomic number by 1. In total the mass number drops by 8 and the atomic number by 3, giving us element the series is named for.
227
89
Ac
which is actinium, the
Binding energy and mass defect
Question 170D: Data Handling
1. 12 6 10
23
= 7.2 10
24
2. There are 7.2 10
27
nuclei in 12 kg of
12
C, so 1 u = 1.66 10
–27
kg.
3. 8.188 10
–14
J; 0.5111 MeV
4. 0.5111 MeV c
–2
5. 934.08 MeV c
–2
; the use of precise values for the electronic charge and the speed of light gives
931.502 MeV c
–2
.
6. You should notice that the total mass of the constituent parts is always greater than the quoted mass of the elements.
7. Hydrogen is an exception as it has no binding energy. Hydrogen, of course, has only one proton
19 Advancing Physics
and no neutron in the nucleus.
8.
E
mc
2 c
2 9 10
16 m
2 s
2 so
m
4200 J / 9 10
16 m
2 s
– 2 or about 4 10
–14
kg – a fractional change that is far too small to be measured.
9. about 2.8 10
–12
kg
10. ½ m v
–2
= 200 kJ (vehicle mass 1 tonne); 2.2 10
–12
kg.
11. Assume 70 kg for the man; change in mass = (10
7
J)/(9 10
16 m
2
s
–2
) = 10
–10
kg fractional change = 1 / 70 of this very small number.
The disappearing Sun
Question 230D: Data Handling
1. Picture the Sun’s radiation spreading out to cover a sphere whose radius is that of the Earth’s orbit. The surface area of this sphere is
A
4 r
2
4 ( 1 .
5 10
11 m )
2
2 .
83 10
23 m
2
.
Each square metre receives 1370 W, so the total power output of the Sun is
P
A
1370 W m
2
3 .
9 10
26
W .
power emitted per tonne
3.9
10
26
W
2
1000
10 30 kg kg
0 .
2 W per tonne less than the power of a typical torch
2. The mass of protons is equal to:
0 .
9 2 10 30 kg 1 .
8 10 29 kg .
The number of protons is:
1 .
8 10
29 kg / 1 .
67 10
27 kg 1 .
1 10
56
.
3. The energy released per proton is
6 .
4 MeV 6 .
4 1 .
6 10
13
J
1 .
02 10
12
J .
The total energy released is
1 .
1 10
56 1 .
02 10
12
J 1 .
1 10
44
The decrease in mass is
J .
20 Advancing Physics
m
E
( c
2
1 .
1 10
44
3 10
8 m
J s
1
)
2
1 .
2 10
27 kg .
(Note that this is about 1 part in a thousand of the Sun’s original mass.)
4. Now we know the amount of energy the Sun releases during its lifetime, and the rate at which it is releasing energy, we can work out how long it will last: lifetime of Sun
1.1
10
44
3 .
9 10
26
J
W
2 .
8 10 17 s or about 10
10
years. So far, the Sun has lasted approximately 4.5 10
9
years, so it might last for another 5.5 10
9
years. The Sun is roughly half-way through its life.
Telling people about risk
Question 20X: Explanation–Exposition
1. Compare your order with others, then justify.
2. Compare your examples with others, then justify.
3. You will want to justify the effectiveness of the presentation.
5. Look for some type of bar chart or other diagrammatic form which can have more impact than a table of numbers.
21 Advancing Physics