I. Composition of Solutions
advertisement
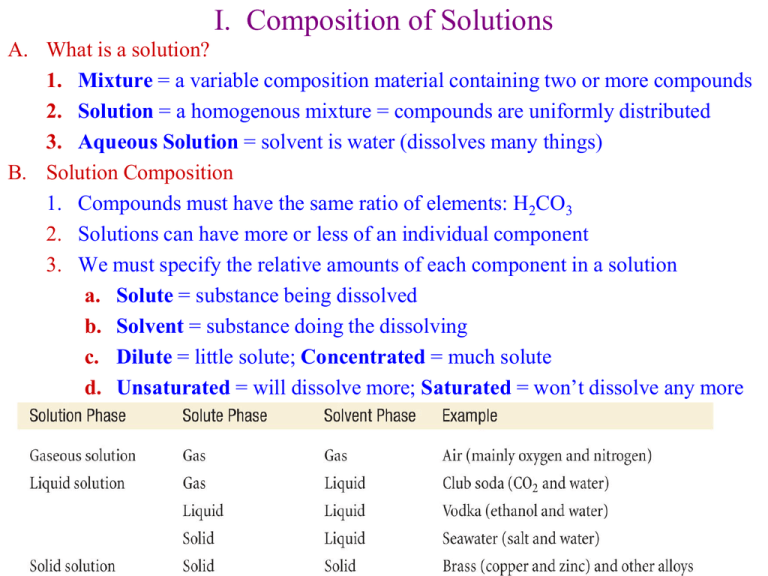
I. Composition of Solutions A. What is a solution? 1. Mixture = a variable composition material containing two or more compounds 2. Solution = a homogenous mixture = compounds are uniformly distributed 3. Aqueous Solution = solvent is water (dissolves many things) B. Solution Composition 1. Compounds must have the same ratio of elements: H2CO3 2. Solutions can have more or less of an individual component 3. We must specify the relative amounts of each component in a solution a. Solute = substance being dissolved b. Solvent = substance doing the dissolving c. Dilute = little solute; Concentrated = much solute d. Unsaturated = will dissolve more; Saturated = won’t dissolve any more C. The role of Entropy in Solution Formation 1. Entropy = amount of disorder in a system = DS a. Chemical systems tend towards more disorder b. Pure Salt and water mix spontaneously c. 2. Two non-interacting (Noble) gases mix spontaneously Entropy will be discussed further in Chapter 17 4. There are several ways to designate the composition of a solution: c a. Example a. 1.00 g ethanol (MW = 46.07 g/mol) b. 100 g water (MW = 18.0 g/mol) 101 mL Molarity, mass percent, mole fraction, molality equivalent s of solute 5. Normality (N) = liter of solution a. Equivalents depends on the reaction of concern b. For Acid-Base Reactions, 1 eq = the amount of a substance giving 1 mole of H+ or OHi. 1 M HCl = 1 N HCl = 1 N H+ ii. 0.5 M H2SO4 = 1 N H2SO4 = 1 N H+ iii. 0.33 M Al(OH)3 = 1 N Al(OH)3 = 1 N OH- c. For Oxidation-Reduction Reactions, 1 eq = amount of a substance giving or accepting 1 mole of electrons i. MnO4- + 5 eMn2+ + 4 H2O ii. 1 M MnO4- = 5 N MnO4- = 5 N eiii. How much MnO4- in 1 N MnO4- ? 1 mol 31.6 g 158 g 1 mol MnO 5 eq eq 4 II. Energies of Solution Formation A. Like Dissolves Like 1. Polar Solvents (H2O) dissolve polar (HF) and ionic solutes (NaCl) 2. Nonpolar Solvents (paint thinner) dissolve nonpolar solutes (paint) 3. Steps for solution formation tell us why Step 1 = Expanding the solute (endothermic) Step 2 = Expanding the solvent (endothermic) Step 3 = Allowing solvent-solute interactions (exothermic) 4. Enthalpy or Heat of Solution = DHsoln is a combination of the 3 steps DHsoln = DHstep 1 + DHstep 2 + DHstep 3 5. Example #1 Oil dissolving in Water (not soluble) a. DHstep 1 is large + because large oil molecules have large intermolecular interaction b. DHstep 2 is large + because highly polar water has large intermolecular forces c. DHstep 3 is small – because nonpolar oil and polar water don’t have very favorable interactions DHsoln = (+large) + (+large) + (-small) = + large (endothermic) 6. Example #2 NaCl dissolving in Water (very soluble) a. DHstep 1 is large + because NaCl has strong (786kJ/mol) bond b. DHstep 2 is large + because of water c. DHstep 3 is large – because Na+ and Cl- interact strongly with water DHsoln = (+large) + (+large) + (-large) = + small (endothermic) d. How does NaCl dissolve if it is slightly endothermic (DH = +)? e. Entropy (DS) = tendency of systems to become disordered i. Disorder is energetically favored over order ii. Disorder will increase in any system unless DH = +Large f. NaCl dissolves in water because DH = +small and Entropy favors it H + O + H + + - H + -O Cl H H H O -O - Na+ + - + H - H + + H H O + H + O H + g. For water solutions DHHyd = DH2 + DH3, so DHsoln = DH1+ DHHyd DHHyd for NaCl = -783 kJ/mol so DHsoln = + 3 kJ/mol III. Factors Affecting Solubilities A. Structure (Polarity; Intermolecular Forces) 1. Polarity depends on structure, and as we have seen, that affects solubility 2. Vitamin examples a. Fat soluble vitamins (nonpolar) i. Primarily C—C and C—H bonds ii. Body fats are also nonpolar and will absorb the vitamins iii. Nonpolar = Hydrophobic = “water fearing” iv. Vitamin A is Hydrophobic and is stored in body fats b. Water soluble vitamins (polar) i. Contain O—H, N—H, and C=O bonds ii. Hydrophilic = “water loving” iii. Vitamin C is hydrophilic, and can’t be stored. B. Pressure 1. Pressure does not affect the solubility of solids or liquids 2. Gas solubility does depend on the pressure 3. CO2 in soda is dissolved at high CO2 partial pressure and then the can is sealed. When you open the can, the partial pressure of CO2 is much less than 1 atm, so the CO2 comes out of solution. 4. Pressure increases the rate of gas molecules entering the solvent. Gas molecules enter faster than exiting until an equilibrium with more dissolved gas is reached. 5. Henry’s Law describes the pressure effects: Sgas = kHPgas a. Pgas = partial pressure of the gas solute b. kH = constant that is specific to each solution c. Sgas = M of the gas d. Concentration of the gas is directly proportional to the pressure e. To obey Henry’s Law, the gas can’t react with the solvent 6. Example: kH = 3.1 x 10-2 mol/L•atm, PCO2 = 5 atm (unopened soda) S kP (0.031mol / L atm)(5atm) 0.16M PCO2 = 4 x 10-4 atm (opened soda) S kP (0.031mol / L atm)(0.0004atm) 1.2 x105 M C. Temperature and Aqueous Solutions 1. Compounds dissolve faster at higher temperatures, but not necessarily more 2. Most solids are more soluble at higher temperatures, some aren’t 3. Must do the experiment with each compound to find out 4. Gases are more soluble at lower temperatures






