N III. Homonuclear Diatomics
advertisement
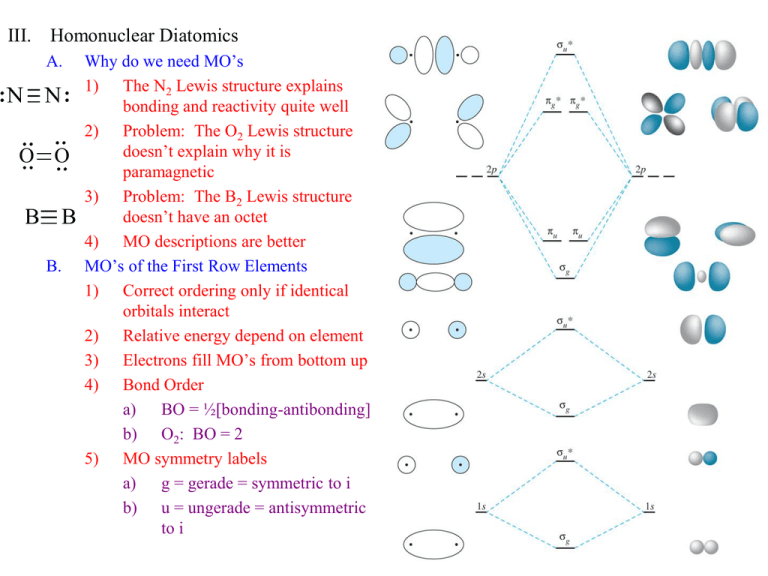
III. Homonuclear Diatomics A. N N O O B B B. Why do we need MO’s 1) The N2 Lewis structure explains bonding and reactivity quite well 2) Problem: The O2 Lewis structure doesn’t explain why it is paramagnetic 3) Problem: The B2 Lewis structure doesn’t have an octet 4) MO descriptions are better MO’s of the First Row Elements 1) Correct ordering only if identical orbitals interact 2) Relative energy depend on element 3) Electrons fill MO’s from bottom up 4) Bond Order a) BO = ½[bonding-antibonding] b) O2: BO = 2 5) MO symmetry labels a) g = gerade = symmetric to i b) u = ungerade = antisymmetric to i C. Orbital Mixing 1) Orbitals of similar energies can interact if symmetry allows 2) This leads to adjustments in the energy ordering of diatomic MO’s a) Example: sg(2s) and sg(2p) can mix lowering sg(2s) and raising sg(2p) b) Example: su(2s) and su(2p) can mix lowering su(2s) and raising su(2p) 3) Effects of mixing a) Energy ordering changes as we move across the first row elements b) Bonding/Antibonding nature of a given MO may change D. MO Theory and Properties of Homonuclear Diatomics 1) Magnetism a) Paramagnetic = strongly attracted by magnetic field due to upaired eb) Diamagnetic = weakly repelled by magnetic field by all paired e2) H2 [sg2(1s)] a) BO = 1 and BL = 74 pm b) H2+ has BO = ½ and is less stable with BL = 106 pm 3) 4) 5) He2 [sg2(1s)su*2(1s)] BO = 0, Noble gas that does not exist as a diatomic Li2 [sg2(2s)] BO = 1 Be2 [sg2(2s)su*2(2s)] BO = 0, not stable 6) B2 [pu1pu1(2p)] a) Paramagnetism of B2 is not explained by Lewis structure B B b) BO = 1 c) Without mixing, all e- paired = diamagnetic d) Mixing makes degenerate pu(2p) lower than sg(2p) e) Filling degenerate orbitals equally (Hund’s Rule) makes it paramagnetic 7) C2 [pu2pu2(2p)] a. BO = 2, mixing suggest that both are p-bonds with no s-bond? b. C2 is rare, but C22- (with a s-bond as well BO = 3) is more stable 8) N2 [sg2pu2pu2(2p)] a. BO = 3 agrees with short strong bond (BL = 109.8 pm, D = 945 kJ/mol) b. After nitrogen the “normal” energy ordering initially predicted returns i. Nuclear charge gets higher as we go across first row ii. Mixing becomes less important because the energy difference between 2s and 2p orbitals increases (s closer to nucleus, p more diffuse) iii. Large energy difference means mixing is less important 9) O2 [sg2pu2pu2pg*1pg*1 (2p)] a. BO = 2, paramagnetic (not explained by Lewis structure) b. O2 ions correlate BO and BL very well O O 10) F2 [sg2pu2pu2pg*2pg*2 (2p)] BO = 1, diamagnetic 11) Ne2 BO = 0 Noble gas, diatomic not stable I. MO’s from d-orbitals d z2 Transition metals and other heavy elements use d-orbitals in their bonding interactions 1) d-orbitals may form s, p, or d bonds a) A s example is the dz2/dz2 interaction b) A p example is the dyz/dyz interaction c) A d example is the dxy/dxy interaction d) d bonds change signs upon C4 rotation around the internuclear axis 2) II. Examples Heteronuclear Diatomics A. Polar Bonds 1) MO pattern is same as homonuclear 2) One set of AO’s will be at a lower energy than the other 3) Valence Orbital Potential Energy a) Negative energies of attraction of e- to the nucleus b) Averaged for all e- on the same level (3p) c) As Z increases left to right, VOPE becomes larger 4) The LCAO for heteronuclear diatomics uses different coefficients because the energies of the 2 atoms are no longer identical a. Y = caYa + cbYb (ca ≠ cb) b. The AO closest in energy to the MO contributes most to it i. In CO the 2s MO is mostly O ii. The 2s* MO is mostly C c. The shape and energy of the MO is similar to the major contributing AO d. e. f. 5) If DE > 12 eV, there is no interaction For CO, BO = 3 Mixing is still important Molecular reactivity occurs at Frontier Orbitals a) HOMO = Highest Occupied Molecular Orbital b) LUMO = Lowest Unoccupied Molecular Orbital C O

