Chemistry Lab Experiment: Limewater and Carbon Dioxide
advertisement

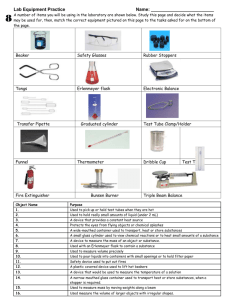
Experiment 1.1 Working in the Chemistry Laboratory (4) Report=163 Introduction: (5) One of the most important components of your chemistry course is the laboratory experience. Although you may have performed experiments in other science courses and may be familiar with some of the laboratory equipment, there is, nevertheless, a great deal more to be learned about the equipment and the safety procedures that are used in a chemistry laboratory. A number of important procedures need to be mastered in order for you to perform the experiments in this course successfully. You will need to know how to use a Tirrill burner, how to filter liquids, how to handle solid chemicals and solutions, and how to heat materials safely. It is important to learn how to make accurate measurements by using graduated cylinders, thermometers, and balances. In addition, you need to learn how to take necessary precautions so that you can work safely in the laboratory. In this introductory experiment, you will have a chance to learn these laboratory techniques as you perform an experiment involving a water solution of calcium hydroxide, Ca(OH)2, commonly called limewater. Limewater is used in a test for the presence of carbon dioxide, CO2, an important product of animal respiration. When carbon dioxide is bubbled through limewater, a cloudy appearance is noted. This cloudiness will eventually settle out and is called a precipitate. Objectives: (2) 1. Demonstrate mastery of essential laboratory techniques and procedures as well as a familiarity with laboratory equipment. 2. Apply laboratory precautions to laboratory procedures. 3. Observe the reaction between limewater and carbon dioxide. 4. Interpret the results of two chemical reactions. 5. Use LoggerPro to determine pH and temperature. Materials: (5) Apparatus: safety glasses laboratory apron Tirrill burner centigram balance filter paper stirring rod 6-mm bent glass tubing 125 mL Erlenmeyer flask 50 mL Erlenmeyer flask one hole stopper ring stand utility clamp Logger Pro thermometer Logger Pro pH spark lighter 250 mL graduated cylinder glycerine hot plate 2-250mL beakers Reagents: 1.0 M HCl, hydrochloric acid magnesium ribbon sodium carbonate calcium hydroxide deionized water Procedure: Part 1 1. Adorn your safety glasses and lab apron. 2. Observe your gas burner. It is a modification of the Bunsen burner, called a Tirrill burner. There is an inlet for the gas, an adjustment for the flow of gas, and an adjustment for the flow of air. A proper mix of air and gas will yield a faint blue flame for maximum heat and minimum Experiment 1-1 soot. Identify the air adjustment and the gas adjustment on your burner. Before lighting the burner, turn the air adjustment to allow as little air as possible. Page 1 slide the tubing gently through the hole of the one hole stopper. 9. Get stamp #2. Part 3 (Make solution and filter) 10. In order to make the limewater solution, you must be able to use a lab balance. A balance must be zeroed before it is used to find masses. The balance is on a flat, stable surface. Find the zero-adjusting button. Press it. 11. Chemicals are measured in a container or on paper, never directly on the pan or platform of a balance. Place a clean, dry weigh boat on the pan of the balance. Press the tare button. 3. Before you light the burner, check to make sure that all students nearby are wearing their safety goggles. Lighting the burner is a one person job. Have a match or spark lighter(striker) ready before you turn on the gas. Light the burner by turning on the gas and holding the spark lighter or lighted match above the barrel of the burner. 4. Adjust the flame to a blue color by changing the flow of air and the flow of gas. If the flame flutters and roars, you have added too much air or not enough gas. 12. Place approximately 1 g of calcium hydroxide in the weigh boat and find it’s mass to the nearest 0.01 g. Record this mass on the Report sheet. 13. Get stamp #3 for massing calcium hydroxide. 14. Add the calcium hydroxide to a clean, dry 250 mL beaker. Use your deionized water bottle to remove all solid from weight boat by squeezing the corners of weight boat together and using the wash bottle to wet the solid and let gravity do its thing so that the solid leaves the weigh boat into the beaker. 15. Use a 250 mL graduated cylinder to measure exactly 175 mL of deionized water 16. Get stamp #4 for graduated cylinder 17. Add the water to the calcium hydroxide in the beaker. 5. Get your first stamp by showing your properly operating burner. 6. Turn off the burner and go on to the next part of the experiment 18. Stir the solution with a stirring rod. Calcium hydroxide is difficult to dissolve. Heating the solution will speed the process. Set the beaker on a hot plate, turn it to “high”, and heat the solution, stirring occasionally (**See Note**), for approximately 5 minutes. Do NOT boil! Not all of the solid will have dissolved, even after heating. Turn off the hot plate. Remove the beaker from the hot plate and place it on the ceramic tile. Let the solution cool and settle for at least five minutes while you set up a funnel to filter the solution. Note: When using a stirring rod, best way to keep uncontaminated is to place it in a small beaker with dionized water. Part 2 7. Obtain a piece of glass tubing that is at 90 degree bend. 8. Find a stopper that has one hole and fits a 125-mL Erlenmeyer flask. Using a drop of glycerine to lubricate the hole in the stopper, Experiment 1-1 Page 2 As shown below 19. The filtration apparatus is shown below. 20. Turn on the vacuum pump. The filter paper should “suck” onto the funnel and make a whistle sound and you can see all the holes through the filter paper. As shown below: Obtain a filter paper from the chemical shelf. Go to a filtration system set-up at different areas around the lab. Make sure rubber stopper is in the Hirsch funnel Place Hirsch funnel into a side arm flask. Make sure the flask is securely fastened to a utility clamp and ring stand. Put rubber hose into the side arm of the flask. Place filter paper into the Hirsch funnel. Make sure all the holes in the funnel are covered up by the filter paper. As shown below: Pour the solution supernate down your stirring rod into the filter paper. Take your deionized water bottle to wet down the filter paper. Make sure all filter paper is wet. Allow the clear solution to filter into the beaker below. Be careful to get the solid on to the filter paper and not on the sides. You do not have to filter the whole solution just enough to get 150 mL. You will use the clear limewater for Parts 4, 5, and 6. Put the filter paper and any solid it may contain into garbage. Wash all other glassware thoroughly with soap and water. Place the clear limewater into a clean 250 mL beaker. Experiment 1-1 Page 3 21. Get stamp #5. Part 4 (Measure pH) pH is a common measurement of solutions. pH measures how acidic a solution is (how much hydrogen ions present). Low pH = many H ions. High pH = low H ions. in and out the pipette. Use a 250 mL beaker to place the DI water. 30. Get Stamp #7. 31. Add the 20 mL of clear limewater into a 50mL Erlenmeyer flask. 32. Obtain a soda straw and gently blow into the limewater through the soda straw. Continue to exhale into the tube until you observe a change. Record your observations on the Report Sheet. 33. Get stamp #8. 22. We will be using LoggerPro sensors to measure pH. Make the pH sensor (see picture) is attached to sensor port. Part 6 34. Rinse the 50 mL flask you used in Part 5 and fill it with 20 mL of fresh, clear limewater as before. 35. In a 125 mL Erlenmeyer flask, place 25 mL of 1M hydrochloric acid. 36. Measure the pH of your acid in the flask and record. Another use of LoggerPro is to measure temperature. 37. Re-open Logger Pro if you closed it 23. Double click on LoggerPro icon on desktop of computer. 24. Both pH and temperature boxes should show up on bottom left. 25. To remove the sensor from its container, just unscrew the cap. DO NOT pull the sensor out from the cap. 26. Clean the sensor by washing it with DI water from squeeze bottle. 27. Place sensor into your 250 mL beacker of clear limewater and wait for the sensor to stop fluctuating. Record pH 28. Clean the pH sensor by swirling the sensor in a 250 mL beaker full of DI water. Then rinse the sensor with DI squeeze bottle. 29. Screw the cap back on and place it back in its upright position. 30. Get stamp #6. Part 5 31. Measure out 20 mL of the clear limewater into the appropriate graduated cylinder. Use a pipette to get exactly 20 mL. Make sure you clean your pipette by squeezing fresh DI water Experiment 1-1 38. Using a thermometer, measure the temperature of the acid and record it on the Report Sheet. Rinse the end of the thermometer with water after removing it from the acid. 39. Obtain your bent tubing from part 2. Obtain a piece of magnesium ribbon approx 5 cm long. Carefully drop the magnesium into the acid and quickly place the stopper with the bent tubing on the top of the flask. Place the open end of the tubing below the surface of the limewater in the 50 mL Flask. Observe what happens and record your observations on the Report Sheet. 40. As soon as the reaction stops, remove the stopper and measure the temperature and pH of the acid. Record it on the Report Sheet. 41. To dispose of ANY excess acid, place about a tablespoon of baking soda to neutralize the acid. Pour out the neutralized acid and place any unreacted magnesium into the trash. Pour the limewater down the drain. Rinse out the glassware. 42. Repeat the same experiment but with no limewater. You will be testing the gas with a fiery splint. 43. Set-up your Tirrell burner. Obtain a wood splint. Obtain the correct amount of magnesium as before. Add the correct about of HCl to the 50 mL Erlenmeyer flask. 44. Light your burner. Set your splint on fire and turn off burner. Page 4 45. Quickly add your magnesium and let react for about 10 seconds. Place splint over the mouth of the flask and record your observations. 50. Dispose your acid as before. Rinse out the glassware. 51. Repeat the same experiment but with no limewater. You will be testing the gas with a fiery splint. 46. Dispose your acid and magnesium as before. Throw splint into the trash. Rinse out the glassware. 52. Set-up your Tirrell burner. Obtain a wood splint. Obtain the correct amount of sodium carbonate as before. Add the correct about of HCl to the 50 mL Erlenmeyer flask. 47. Place 20 mL of limewater in the 50 mL Flask as done before 48. Place 35 mL of 1 M HCl in the 125 mL flask. Obtain ≈2 grams of sodium carbonate. Carefully transfer the sodium carbonate into the 125 mL flask and quickly place the stopper with the bent tubing on the top of the flask. Place the open end of the tubing below the surface of the limewater in the 50 mL Flask. Observe what happens and record your observations on the Report Sheet. 53. Light your burner. Set your splint on fire and turn off burner. 54. Quickly add your solid and place splint into the mouth of the flask and record your observations. 55. Dispose your acid as before. Throw splint into the trash. Rinse out the glassware, followed by rinsing 3 times with 10 mL of deionized water. 49. As soon as the reaction stops, remove the stopper and measure the temperature and pH of the acid. Record it on the Report Sheet. 56. Get stamp #9. 57. Before leaving the laboratory, clean up all materials and wash your hands Data and Observations: (108) Part 1 (10) Stamp 1......................... Part 2 (10) Stamp 2......................... Part 3 (2) mass of calcium hydroxide g (10) Stamp 3......................... (10) Stamp 4......................... (10) Stamp 5......................... Part 4 (2) pH of limewater (10) Stamp 6......................... Part 5 (10) Stamp 7......................... Observations of blowing into limewater. (10) Stamp 8......................... Experiment 1-1 Page 5 Part 6 (14) Data: HCl / Mg Reaction HCl / Na2CO3 Reaction pH of Acid before reaction Temperature before reaction Temperature after reaction pH after reaction Reaction description Limewater description Splint Test (10) Stamp 9......................... Analysis and Conclusions: (23) (6) 1. In part 6, was carbon dioxide produced in either of the reactions? How do you know? (6) 2. In part 6, was hydrogen gas produced in either of the reactions? How do you know? (3) 3. What three observations in part 6 lead you to believe that chemical reactions occurred? (2) 5. Why was it necessary to filter the cloudy limewater prior to use in parts 4, 5, and 6? (2) 6. Why is it necessary to use a weigh boat or some other container when you find the mass of solids? (two reasons) (2) 7. Based on the pH, is limewater acidic or basic? (2) 4. Why was the limewater solution you made in part 3 cloudy? Synthesis: (16) (2) 1a. If you were to find the mass of the hydrochloric acid and the mass of the magnesium strip before the reaction, how would that mass compare with the mass of the material remaining in the flask after the reaction was complete? (2) 4. Many antacids are carbonate or bicarbonate compounds. One manufacturer used to refer to the “burp of relief” when a person took the antacid. Explain what is meant by “burp of relief” and what is reacting with the antacid. (2) 1b. If you were able to contain the gas produced (rather than let it escape as you did), how would that procedure affect the masses before and after the reaction? (4) 2. A number of SI units were used in this experiment. Review the procedure and make a list of all of the units you used in your measurements. (6) 3. Matter in three different phases was observed in this experiment. Give two examples from the experiment of each phase. solid: ______________________ liquid: ______________________ gas: _______________________ Experiment 1.1 Page 6