Biology 242 - Lab LAB #9 (9
advertisement

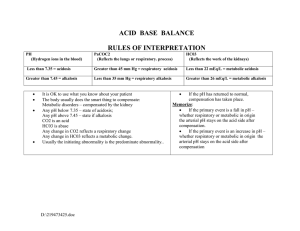
Biology 242 - Lab LAB #9 (9th/19 Lab Sessions for Winter Quarter, 2008) TOPICS TO BE COVERED: DESIRED OUTCOMES: After completing the activities described for this lab session, students should: MATERIALS DESIRED: The Respiratory System SPIROMETRY A spirometer is an instrument used to measure different volumes of air involved in breathing. The Phipps and Bird Wet Spirometer is based on the simple mechanical principle that air, exhaled from the lungs, will cause displacement of a closed chamber which is partially submerged in water. Basically, the Spirometer consists of two vessels: a larger vessel containing water and having a breathing hose attached to it; and a smaller vessel inverted and suspended in the water. A counterweight and indicator are attached to the inverted chamber. Air blown into the inverted chamber will cause it to rise, thus moving an indicator arrow along the horizontal scale, which is calibrated in liters, to give lung volume measurements. Experiment 1: Measuring TIDAL VOLUME Measure the amount of air exhaled or inhaled during normal, quiet breathing (TV) The student should sit by the spirometer, breathing quietly and normally for about a minute. After inhaling a normal breath, places the mouthpiece between the lips (get a good "seal") and exhale in a normal, unforced way, into the spirometer mouthpiece. The volume should be read and recorded from the horizontal scale. Experiment 2: Measuring EXPIRATORY RESERVE VOLUME Measure the amount of air that can be forcibly breathed out after normal expiration (ERV) The student stands, breathing normally for a minute or so, then, after a normal exhalation puts the mouthpiece between the lips, and forcibly exhales all the additional air possible. Experiment 3: Measuring INSPIRATORY RESERVE VOLUME Measure the amount of air that can be inhale following normal TV inhalation (IRV) Standing, the student breathes normally for a minute; then breathes as deeply as possible. With the mouthpiece inserted, the student then exhales normally, without forcing the air out. The IRV reading is obtained by subtracting the student's TV from the reading recorded on the spirometer. Experiment 4: Measuring VITAL CAPACITY Measure the maximum amount of air which can be forcibly exhaled immediately following a maximal inhalation (VC) (VC = TV + IRV + ERV Standing, the student slowly and deeply breathes in and out for awhile, then breathes in as deeply as possible, places the spirometer mouthpiece in position, and breathes out as forcibly as possible. In addition to the experiments described above, four other measurements of lung capacity should be calculated: Residual Volume (RV), Functional Residual Capacity (FRC), Inspiratory Capacity (IC), and Total Lung Capacity (TLC). Residual Volume (RV): The lungs are never completely emptied, always containing about 1200 ml of air in adults. Obviously, this measurement cannot be obtained by conventional spirometry. Functional Residual Capacity (FRC): This is the amount of air remaining in the lungs after normal exhalation, FRC = ERV + RV. Inspiratory Capacity (IC): The amount of air which can be inhaled after normal expiration, IC = TV + IRV. Total Lung Capacity (TLC): This is the amount of air contained in the lungs after a maximal inhalation, TLC = TV + IRV + ERV + RV. SPIROMETRY PROBLEMS: 1. Given: VC = 4300 ml IC = 3000 ml TV = 400 ml RR = 16/min Calculate: a) IRV b) ERV c) MVR 2. Given: TV = 400 ml IRV = 2600 ml ERV = 1000 ml RR = 12/min Calculate: a) VC b) IC c) MVR 3. Given: IRV IC RV VC Calculate: = 2800 ml = 3250 ml = 1200 ml = 5375 ml a) TV b) ERV c) TLC Biology 251 SPIROMETRY LAB – Page Two I. SOME BACKGROUND INFORMATION ON ACIDOSIS and ALKALOSIS: Recall that the normal pH value for body fluids is between 7.35 – 7.45. When the pH value of body fluids falls below 7.35, the condition is called acidosis; and when the pH value is above 7.45, the condition is called alkalosis. Metabolism produces acidic products that lower the pH of body fluids. For example, carbon dioxide is a byproduct of metabolism, and carbon dioxide combines with water to form (carbonic acid CO2 + H2O → HCO3-). Also, lactic acid is a product of anaerobic metabolism, protein metabolism produces phosphoric and sulfuric acids, and lipid metabolism produces fatty acids. These acidic substances must continuously be eliminated from the body to maintain pH homeostasis. Rapid elimination of acidic products of metabolism may result in alkalosis*, however; and the failure to eliminate acidic products of metabolism results in acidosis**. **The major effect of acidosis is depression of the central nervous system (CNS). When the pH of the blood falls below 7.35, the CNS malfunctions, and the individual becomes disoriented and possibly comatose as the condition worsens. **The major effect of alkalosis is hyperexcitability of the nervous system. Peripheral nerves are affected first, resulting in spontaneous nervous stimulation of muscles. Spasms and tetanic contractions and possibly extreme nervousness or convulsions result. Severe alkalosis can cause death as a result of tetany of the respiratory muscles. Although buffers in body fluids help resist changes in the pH of those body fluids, the respiratory system and the kidneys are the main regulators of pH in the body fluids. Malfunctions of either the respiratory system or the kidneys can result in acidosis or alkalosis. Acidosis and alkalosis are categorized by the CAUSE of the condition. Respiratory acidosis or respiratory alkalosis results from abnormalities of the respiratory system which, in turn, allow too much CO2 to be retained or too much CO2 to be eliminated. Metabolic acidosis or metabolic alkalosis results from all causes other than abnormal respiratory functions. Inadequate ventilation of the lungs causes respiratory acidosis (see Table below), because the rate at which CO2 is eliminated from the body fluids through the lungs decreases. The result of this is an increase in the concentration of carbon dioxide in body fluids. As carbon dioxide levels increase, excess carbon dioxide reacts with water to form carbonic acid. The carbonic acid then dissociates to form hydrogen ions (H+) and bicarbonate ions (HCO3-) as per the following (reversible) reaction: H2CO3 → H+ + HCO3-. The increase in H+ concentration causes the pH of body fluids to decrease. If the pH of body fluids falls below 7.35, symptoms of respiratory acidosis become apparent. TABLE: Clinical Observations of Acidosis and Alkalosis ACIDOSIS: ■Respiratory acidosis ▪Reduced elimination of CO2 from body fluids ▪Asphyxia ▪Severe emphysema ▪Advanced asthma ▪Hypoventilation (ie, impaired respiratory center function due to ■Metabolic acidosis trauma, tumor, shock, or renal failure ▪Elimination of large amounts of bicarbonate ions resulting from mucous secretion (ie, severe diarrhea and/or vomiting of lower intestinal contents) ▪Direct reduction of body fluid pH as acid is absorbed (ie, ingestion of acidic drugs such as aspirin) ▪Production of large amounts of fatty acids/other acidic metabolites such as ketone bodies (ie, untreated diabetes mellitus) ▪Inadequate oxygen delivery to tissues resulting in anaerobic respiration and lactic acid build-up (ie, exer________________cise, heart failure, or shock)_____________________________________________________ ALKALOSIS: ■Respiratory alkalosis ▪Reduced CO2 levels in the extracellular fluid ▪Hyperventilation due to emotions (ie, fear, anxiety, trauma reaction) ▪Decreased atmospheric pressure due to high altitudes (reduces blood O2 levels); stimulates hyperventilation ■Metabolic alkalosis ▪Elimination of H+ and reabsorption of HCO3- in the stomach or kidney (ie, severe vomiting or formation of acidic urine in response to excess aldosterone) ▪Ingestion of alkaline substances (ie, large amounts of sodium bicarbonate (“antacids” such as TUMS, etc.) ____________________________________________________________________________________________ Biology 251 SPIROMETRY LAB – Page Three SELF-TEST MATCHING: Select the letter that best describes the acid—base imbalance for each condition listed. ______1. Diarrhea ______2. Oral intake of excessive NaHCO3 ______3. Emphysema A. Respiratory acidosis ______4. Early stage of overdose of aspirin B. Metabolic acidosis ______5. Vomiting C. Respiratory alkalosis ______6. Diabetes mellitus D. Metabolic alkalosis ______7. Pneumonia ______8. Anxiety MULTIPLE CHOICE ______9. The most accurate method of determining pH is to A. use litmus paper. C. use a pH meter. B. use pH or nitrazine paper. D. use a spectrophotometer. _____10. A solution that has the same number of H+ as OH- is A. neutral. C. basic. B. acidic. D. salty. _____11. If one solution is 1000 x as acidic as another, how many pH units would there be between the two? A. one C. three B. two D. four _____12. A person with an arterial blood pH of 7.62 would be in a state of A. acidosis. C. neutrality. B. alkalosis. D. death. _____13. The organs primarily responsible for maintaining acid-base balance in the body are the A. liver and spleen. C. kidneys and heart. B. liver and lungs. D. lungs and kidneys. _____14. Which of these would be formed if a strong acid were added to a weak base in a buffered solution? A. A weak acid C. A strong base B. water D. Both a weak base and a strong acid _____15. Which of these acids is the strongest? A. 0.01M HCl C. 1 M NaOH B. 1 M HCl D. 0.05M HCl _____16. What effect does breathing in and out of a paper bag have on a person’s arterial pCO2 level? A. The level decreases. C. It has no effect. B. The level increases. D. The level alternately increases and decreases . _____17. The strength of an acid refers to A. the number of H+ in each molecule. C. the degree to which its molecules ionize in water. B. the type of inorganic salt it forms. D. the concentration of acid molecules. _____18. The most common buffer in plasma and intercellular fluid is A. bicarbonate. C. hemoglobin. B. phosphate. D. sodium and potassium. TRUE / FALSE _____19. The pH of blood is constant. _____20. The pH of venous blood is usually lower than that of arterial blood. _____21. Buffer solutions neutralize acidic or alkaline solutions. _____22. Phosphate buffers may act as either acids or bases. _____23. More drops of acid are required to change the pH of a buffered solution than a non-buffered solution. _____24. The respiratory system provides the major compensatory mechanism for respiratory acidosis. _____25. A base is a hydroxyl ion acceptor. DISCUSSION QUESTION: 26. CO2 itself is not an acid; but, from the standpoint of respiratory physiology, it is considered an acid. Why?