AP Chemistry Allotropes of Carbon
advertisement
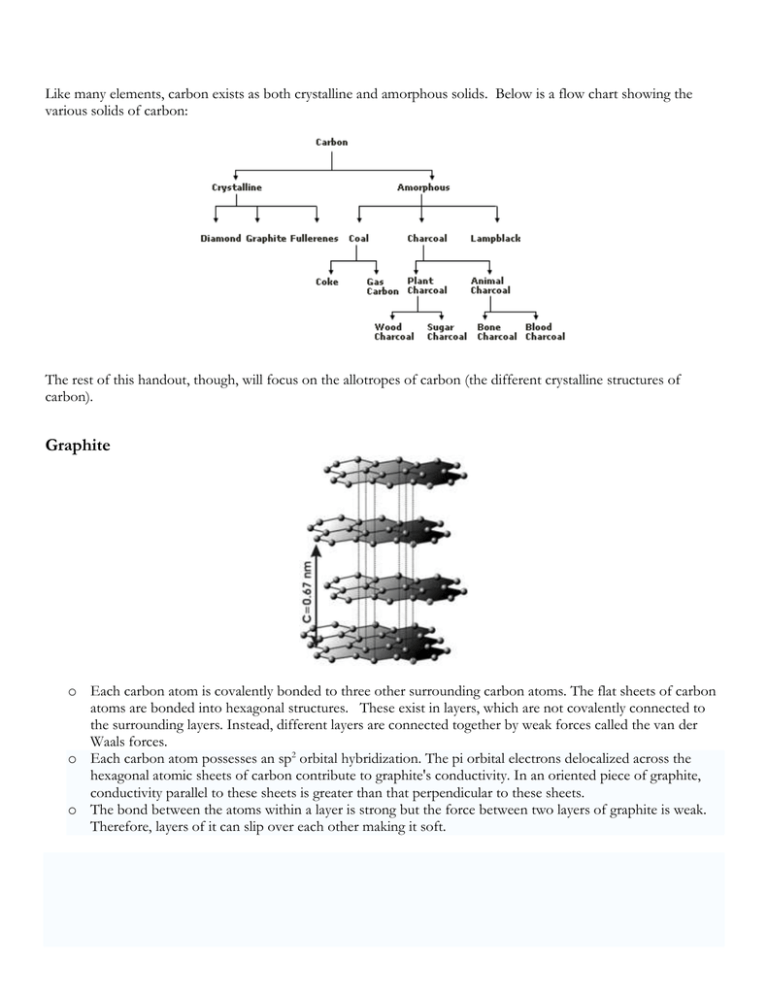
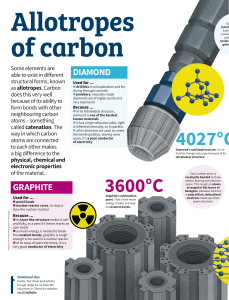
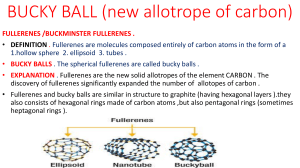
Like many elements, carbon exists as both crystalline and amorphous solids. Below is a flow chart showing the various solids of carbon: The rest of this handout, though, will focus on the allotropes of carbon (the different crystalline structures of carbon). Graphite o Each carbon atom is covalently bonded to three other surrounding carbon atoms. The flat sheets of carbon atoms are bonded into hexagonal structures. These exist in layers, which are not covalently connected to the surrounding layers. Instead, different layers are connected together by weak forces called the van der Waals forces. o Each carbon atom possesses an sp2 orbital hybridization. The pi orbital electrons delocalized across the hexagonal atomic sheets of carbon contribute to graphite's conductivity. In an oriented piece of graphite, conductivity parallel to these sheets is greater than that perpendicular to these sheets. o The bond between the atoms within a layer is strong but the force between two layers of graphite is weak. Therefore, layers of it can slip over each other making it soft. Diamond o Each carbon atom is covalently bonded to four other surrounding carbon atoms. A three dimensional network of tetrahedral linkages make a diamond one large network covalent molecule. o Each carbon is sp3 hybridized. With no unhybridized orbitals or delocalized electrons, diamond is a nonconductor of heat or electricity. o The network covalent 3-D covalent bonded structure makes diamond a very, very hard substance Fullerenes (Buckminsterfullerenes, Buckyballs) o Any of a class of closed, hollow, aromatic carbon compounds that are made up of 12 pentagonal and differing numbers of hexagonal faces. Fullerenes consist of even numbers of sp2 linked carbon atoms, with a range of from 32 to as many as 600 atoms. Fullerenes are the third form of pure carbon known to exist, after the network solids of diamond and graphite. o This crystalline structure is different from the diamond or graphite crystal in that distinct molecules form the unit cell of the crystal. The C60 molecules are arranged into a face-centered-cubic unit cell. The sides of this cubic cell measures 14 Angstroms. Each C60 molecule have a diameter of 10 Angstroms. The molecules are held together in the crystal by weak Van der Waals forces. o Fullerenes derive their name from the American architect R. Buckminster Fuller, whose geodesic dome design is similar to the molecular structure of C60. Buckminsterfullerene, or buckyball, is the name applied to C60 itself. o The unique structure and properties of buckminsterfullerene suggest potential uses for fullerenes as superconductors, lubricants, industrial catalysts, and drug-delivery systems (e.g., targeted cancer therapy).