AP Chemistry Kinetics Sample Problems – Rate Laws
advertisement

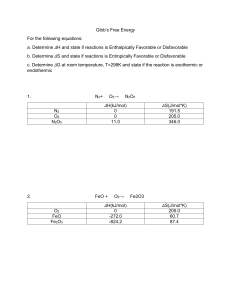
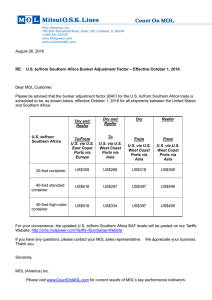
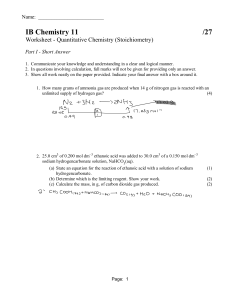
AP Chemistry Kinetics Sample Problems – Rate Laws 1. At 25 oC, hydrogen iodide breaks down very slowly to hydrogen gas and iodine vapor with a rate constant of 2.4 x 10-21 L/mol.s. If 0.0100 mol of HI(g) is placed into a 1.0 L container at 25 oC, how long will it take for the concentration of HI to reach 0.00900 mol/L? 2. Given the following equation N2O5(g) → N2(g) + 5/2 O2(g) and the following plots Determine the order of the reaction with respect to N2O5 3. Iodine-123 is used to study thyroid gland function. This radioactive isotope breaks down in a first order process with a t½ = 13.1 hours. What is k for this process? 4. A first order reaction A → B is 38.5% complete in 480 seconds. a. Calculate the rate constant b. What is the value of the half-life? c. How long will it take for the reaction to go to 95% completion?