Chem 101 Introduction to Inorganic Chemistry A. Unit conversions
advertisement

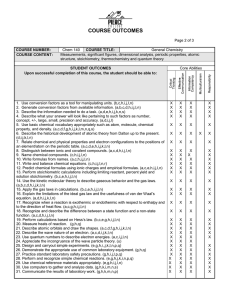
COURSE OUTCOMES COURSE NUMBER: COURSE CONTENT: A. Unit conversions B. Atomic structure C. Periodic properties D. Chemical bonds E. Basic stoichiometry Chem 101 COURSE TITLE: Introduction to Inorganic Chemistry F. States of matter G. Solutions H. Equilibrium J. Acids and bases K. Oxidation/reduction reactions. STUDENT OUTCOMES Upon successful completion of this course, the student should be able to: 1. 2. 3. 4. Use conversion factors as a tool for manipulating units. (b, c, h, i, j, l, n) Generate conversion factors from available information. (a, b, c, d, h, i,j l, n, s) Describe the information needed to do a task. (a, d, e, h, i, j, k, l, n) Describe what your answer will look like pertaining to such factors as number, concept, +/-, large, small, precision, and accuracy. (a,d,i,j,l) 5. Use basic chemical vocabulary appropriately such as atom, molecule, chemical property, and density. (a,c,d,f,g,h,i,j,k,l,m,n,q,t) 6. Relate chemical and physical properties and electron configuration to the position of an element/atom on the periodic table. (a,c,d,e,h,i,j,k,l,n) 7. Distinguish between ionic and covalent compounds. (a,c,d,h,i,j,l,n) 8. Name chemical compounds. (c,h,i,j l,n) 9. Write formulas from names. (a,c,h,i,j,l,n) 10. Write and balance chemical equations. (c,h,i,j,l,n,t) 11. Predict chemical formulas using ionic charges and empirical formulas. (b,c,e, h,i,j,l,n) 12. Perform stoichiometry using mol/mol and mol/gram relationships. (a,c,e,h,i j,l,n) 13. Describe and explain gaseous behavior. ( a,c,d,g,i,m,n,q) 14. Describe s and p atomic orbitals and draw the shapes. (a,c,d,f,g,h,i,j,k,l,n) 15. Describe the states of matter and the factors that affect the transitions between one state an another. (a,c,d,f,g,h,i,j,k,l,m,n) 16. Explain how intermolecular forces affect the physical properties of matter. (a,c,d,e,f,g,h,i,j,k,l,n,o,r,s) 17. Describe solution processes including writing equations for reactions that occur in solutions. (a,c,d,e,f,g,h,i,k,l,m,q,s) 18. Identify oxidation-reduction reactions. (a,c,d,e,f,g,i,j,l,m,o,p) 19. Perform calculations related to the preparation of solutions involving molarity, gram-percent and dilution. (a,b,c,d,e,f,g,h,i,j,l,m,n,s) 20. Relate tonicity of solutions to movement across semi-permeable membranes. (a,c,d,e,f,g,h,i,k,l,m,n,q,r,s,t) 21. Complete and balance neutralization reactions. (a,c,e,g,h,i,l,m,q) 22. Identify acids and bases. (a,c,d,e,f,g,h,i,j,k,l,o,p,s) 23. Demonstrate an understanding of pH by relating it to hydrogen ion concentration and hydroxide ion concentration. (a,b,c,d,e,f,g,h,i,j,k,l,m,n,s) 24. Describe and explain the formation and function of a buffer system. (a,c,d,f,g,h,i,l,m,n,r,s) 25. Describe and explain the factors that affect the rate of reaction. (a,c,d,f,g,h,i,j,k,l,n,q,r,s,t) 26. Describe the relationship between energy changes and chemical processes. (a,c,d,f,g,h,I,r,s) 27. Describe equilibrium and relate it to La Chatelier and the size of equilibrium constant. (a,c,d,f,g,h,i,j,k,l,m,n,q,r,s,t) 28. Describe and carryout simple experiments. (e,g,h,i,j,k,l,n,p,q,) 29. Use common laboratory equipment appropriately. (g,h,q) 30. Practice standard laboratory safety precautions. (g,h,i,j,l,p,q) 31. Perform and recognize simple chemical reactions. (e, g, h, j, k, l, n, p, q) 32, Use chemical reference material appropriately. (e, g, h, i,j,l,n) 33. Communicate the results of laboratory work, including calculations and graphs. (g,h,k,m,n,p) Core Abilities X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X METHODS AND TOOLS FOR ASSESSMENT: a. conceptual questions b. computational questions c. multiple choice questions d. essay questions e. identifying unknowns f. classroom observation g. laboratory observation h. lab reports i. in-class group assignments j. extended group assignments k. oral presentation l. individual assignments m. classroom participation n. written reports o. self-evaluation p. peer-evaluations q. demonstrations r. one minute papers s. concept maps t. role playing