Laboratory #3 Differentiation of Staphylococcus Skills= 20 Points
advertisement

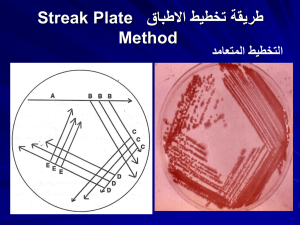
LABORATORY # 3 Differentiation of Staphylococcus sp. Laboratory #3 Differentiation of Staphylococcus Skills= 20 Points Objective: At the completion of this activity, the student will be able to: 1.Correctly differentiate Staphylococcus aureus, Staphylococcus epidermidis and Staphylococcus saprophyticus through the use of colony morphology, catalase test, hemolytic properties, gram stain, coagulase tests, Staphyloslide and Novobiocin sensitivity. Materials: Blood agar cultures of: Staphylococcus aureus Staphylococcus epidermidis Staphylococcus saprophyticus 3% hydrogen peroxide (Catalase reagent) 2-Microscope slides Microscope Immersion oil Coagulase plasma Gram stain materials 1-Staphyloslide 2-Novobiocin disks 1-TSA References: 1. Mahon and Manuselis, Textbook of Diagnostic Microbiology, Fourth Edition, Chapter 14 2. BD BBL Coagulase Plasma package insert 3. BD BBL Staphyloslide package insert Principles: Bacteria in the genus Staphylococcus are gram positive spherical cells that occur singly, occasionally in pairs, but most frequently in irregular clumps. The appearance of a gram stained smear is usually sufficient to distinguish staphylococci from the streptococci because of the characteristic cell grouping (grape-like clusters) of staphylococci. These two groups may also be distinguished by the presence of the enzyme catalase, S.aureus being catalase positive and Streptococcus catalase negative. MLAB 2534 – Laboratory 3 – Page 1 LABORATORY # 3 Differentiation of Staphylococcus sp. Staphylococci are normal flora on the skin and mucous membranes, but can cause infection under certain circumstances. S.aureus is more pathogenic than the other common members of the genus, S.epidermidis and S.saprophyticus. Disease processes with S.aureus are numerous. The portal of entry is variable, since they gain access to the body via the skin, the respiratory tract or the genito-urinary tract. Some of the infections of humans in which S.aureus is the etiological agent are boils, carbuncles, impetigo, meningitis, osteomyelitis, urinary infections, and food poisoning. S.epidermidis has been known to cause various hospital-acquired infections (such as prosthetic or indwelling devices), whereas S.saprophyticus is mainly associated with urinary tract infections in young females who are sexually active. S.aureus can be differentiated from S.epidermidis and other gram positive cocci by the following S. aureus characteristics: Test(s) Pigment Hemolysis Catalase Coagulase Novobiocin Mannitol S = Sensitive R = Resistant S. aureus Gold-white + + + S + S. epidermidis White-yellow + S - S. saprophyticus White-yellow + R - Procedure: Working in Pairs: 1. Colony Morphology Observe individual colonies of S.aureus, S.epidermidis and S.saprophyticus. Record the colony morphology and color of each on the chart at the end of this exercise. 2. Hemolytic Properties Some bacteria synthesize the enzyme hemolysin. Hemolysin is an exoenzyme that lyses red blood cells. If a colony of bacterial cells is producing hemolysin and secreting it into the medium, there will be a round, clear zone surrounding the colony because the red blood cells in that area have been lysed (zone of hemolysis). S.aureus is usually hemolytic, but sometimes it is not. The presence or absence of hemolytic properties, therefore, cannot be used as a definitive identification of Staphylococcus species. a. Observe the blood agar plates of S.aureus, S.saprophyticus and S.epidermidis; note any zone of hemolysis around well-isolated colonies. Report results as hemolytic, or non-hemolytic. b. Record your observations in the appropriate columns of the chart. MLAB 2534 – Laboratory 3 – Page 2 LABORATORY # 3 Differentiation of Staphylococcus sp. 3. Gram Stain As previously mentioned, species of Staphylococcus and gram positive cocci may RARELY occur singly, occasionally in pairs, but most often in clusters. a. Prepare smears of S.epidermidis and S.saprophyticus. Use your smear from Lab #2 for S.aureus. b. Carefully gram stain each slide, EXCEPT the S.aureus smear, and allow to air dry or blot gently with bibulous paper. c. Under oil immersion, observe the gram reaction, morphology and arrangement of cells for each organism. 2. Record your observations. 4. Catalase Test Staphylococcus species contain the enzyme catalase, whereas most species of Streptococcus (another gram positive coccus) do not. Catalase will break down hydrogen peroxide. When mixed with 3% hydrogen peroxide, catalase positive organisms will generate bubbles of oxygen which are visible to the naked eye. Catalase negative organisms do not. It is preferable to test colonies from media without blood since erythrocytes possess catalase activities. In addition, be careful not to gouge agar when picking up colonies for this same reason. H2O2 → H2O + O2 (gas bubbles) a. Test S. aureus, S.epidermidis and S.saprophyticus for catalase activity. For each, use a clean slide. With a loop or wood applicator stick, transfer cells from the center of a well-isolated colony to the surface of the slide. b. Add 1 or 2 drops of 3% hydrogen peroxide. a. Rapid appearance and sustained production of gas bubbles or effervescence is indicative of a positive test. Since some bacteria may possess enzymes other than catalase that can decompose hydrogen peroxide, a few tiny bubbles forming after 20 to 30 seconds is not considered a positive test. c. Record results of each organism in the chart. A positive result will be reported as “positive,” whereas as negative result (no bubbles) will be reported as “negative.” 5. Coagulase Test Coagulase is an exoenzyme that causes fibrin of blood plasma to clot. Pathogenic S.aureus produces coagulase, while non-pathogenic strains are coagulase negative. Two forms of coagulase may be produced by S.aureus: free and/or bound. Bound coagulase, also known as clumping factor, is attached to the cell wall of the organism. Free Coagulase is an intracellular enzyme produced when the organism is MLAB 2534 – Laboratory 3 – Page 3 LABORATORY # 3 Differentiation of Staphylococcus sp. cultured in broth. The slide test is simple to perform and rapid, but detects bound coagulase only. Therefore, all negative slide coagulase must be followed by a tube test, which will detect both bound and free coagulase. The test tube test is more sensitive because it can pick up smaller quantities of coagulase. In the tube test procedure, free coagulase is liberated from the cell which acts on prothrombin in coagulase plasma. This product then acts on fibrinogen to form a clot. In this exercise perform a slide and tube coagulase test on both organisms, regardless of the results of the slide test. a. Rapid Slide Test i. For each organism, use a clean glass slide. Place one drop of coagulase plasma on each. Emulsify a loopful of the colonies (approximately 2-4) to be tested in the drop on the appropriate slide. Colonies should be from an 18-24 hour old culture. ii. Mix with the loop or wood applicator stick to obtain a smooth suspension. Gently rock the slide. iii. If the test is coagulase positive, visible clumps will appear within 1 to 2 minutes. It may be necessary to observe the mixture over a lamp to see the clumping appearance. A positive result is notated as a “positive,” no grading is required. A positive result would also be interpreted as S.aureus on the patient report form because S. aureus is the only Staphylococcus that is coagulase positive. iv. If no agglutination is observed, it will be reported as “negative.” v. Discard slides in a biohazard waste container and record results for each organism. b. Tube Test i. For each organism which had a negative rapid slide test, perform the tube test. ii. For each organism, label a small test tube with patient name and ID number. Place approximately 0.5 ml of coagulase plasma into each tube. iii. Inoculate the plasma with a large loopful of the colonies to be tested. iv. Incubate the tubes in a 37°C incubator or waterbath. Coagulase positive organisms usually produce a visible clot within 1-4 hours. Examine the tubes periodically by gently tipping the tube. DO NOT shake or agitate the tube which would cause dissolution of the clot. A positive result is notated as a “positive,” no grading is required. A positive result would also be interpreted as S.aureus on the patient report form because S.aureus is the only Staphylococcus that is coagulase positive. v. After the 1-4 hour incubation time, if the specimen is still negative, parafilm the tubes and reincubate for 24-48 hours and observe MLAB 2534 – Laboratory 3 – Page 4 LABORATORY # 3 Differentiation of Staphylococcus sp. again for clot formation. vi. After 24 hours, if the absence of a clot is observed, report as ‘negative”. A negative result would also be interpreted as Coagulase-negative Staphylococcus on the patient report. 6. Staphyloslide This procedure is a latex slide agglutination test for the differentiation of staphylococci that possess clumping factor and/or Protein A (found in S. aureus). This kit consists of blue latex particles coated with human fibrinogen and IgG. Once the latex reagent is mixed with colonies, Staph that have protein A or clumping factor will cross-link giving a visible agglutination. a. Mix the latex reagent by gentle inversion. b. Label the test circle with patient or organism name. c. Dispense 1 drop of the test latex onto one circle on the reaction card and one drop of Control latex onto another circle. d. Using 3-5 isolated colonies of S.aureus from a nonselective media, such as blood agar, combine with the test latex reagent, spreading the solution over the entire test circle. e. Using 3-5 isolated colonies of S.aureus from a nonselective media, such as blood agar, combine with the control reagent, spreading the solution over the entire control circle. f. Hand rock the card for 20 seconds. Observe for agglutination by reading macroscopically. i. A positive result is regarded as agglutination within 20 seconds with no agglutination in the control circle. This indicates the presence of S. aureus. A negative result is obtained if no agglutination occurs and a smooth suspension remains at 20 seconds in the test circle. A negative reaction would be reported on the patient chart as Coagulase-negative Staphylococci. g. Dispose of reaction circle in appropriate biohazard container. 7. Novobiocin Susceptibility a. Label the blood agar plate with organism or patient name, student name, and date. b. Streak S. epidermidis and S. saprophyticus on a split blood agar plate to obtain a good lawn of growth. c. Place a 5 µg novobiocin disk in the center of each inoculum. d. Incubate at 37°C for 18 to 24 hours. e. Examine the plate for inhibition around the disk. Measure the zone diameter in millimeters and record results. The diameter should be read at the place of complete inhibition, disregarding colonies that are only detected by close scrutiny or by using transmitted light. a. Susceptible zone: > 12 mm b. Resistance: equal to or lesser than 12 mm. MLAB 2534 – Laboratory 3 – Page 5 LABORATORY # 3 Differentiation of Staphylococcus sp. f. Interpret whether the organism is susceptible or resistant using the zone sizes, but for reporting purposes use either “S” (susceptible) or “R” (resistant) in the report form. a. S. saprophyticus is resistant; S. epidermidis is sensitive. Quality Control Quality control ensures that the information generated by the laboratory is accurate, reliable, and reproducible. This is accomplished by assessing the quality of the specimens, monitoring the performance of test procedures, reagents, media, and personnel. For the reagents used in this lab, QC should be performed weekly and on each new lot or shipment of the product using ATCC control organisms. Results should be reported in the QC log. Components of kit tests should not be interchanged with those from another kit. Technical Notes Hydrogen peroxide (Catalase) is subject to degradation, especially if exposed to light. The reagent should be kept in an amber-colored container and checked daily against a known positive and negative control. It should never be assumed that an unknown organism is a gram positive coccus without performing a gram stain in addition to a Catalase test. Other catalase-positive bacteria can resemble staphylococci, in particular yeasts. Do not perform differentiation tests on a mixed culture, wait for isolated colonies. For the purpose of this lab, the student is exposed to multiple methods to identify whether the organism produces coagulase. However, in the clinical setting, usually only one method, such as a kit test is used. MLAB 2534 – Laboratory 3 – Page 6 LABORATORY # 3 Differentiation of Staphylococcus sp. Report Form: Staphylococcus Differentiation Points= 20 Name ___________________________________ Date _______________________ Test S. aureus S. epidermidis S. saprophyticus Colony Morphology Hemolytic Properties Gram Stain & Morphology Catalase Rapid Slide Coagulase Tube Coagulase Staphyloslide Novobiocin Sensitivity MLAB 2534 – Laboratory 3 – Page 7 LABORATORY # 3 Differentiation of Staphylococcus sp. Lab #3: Staphylococci Biochemical Tests and Media Study Questions Points: 12 Name:__________________________ Biochemical Test or Test Principle Media Date:_____________________ Explain how this test is used to identify the Staphylococci Gram Stain Catalase Production MLAB 2534 – Laboratory 3 – Page 8 LABORATORY # 3 Differentiation of Staphylococcus sp. Biochemical Test or Test Principle Media Coagulase slide test Explain how this test is used to identify the Staphylococci Coagulase tube test Staphyloslide Novobiocin resistance MLAB 2534 – Laboratory 3 – Page 9