Attachment G. – Vertebrates, Invertebrates, or Plants Research Organisms General Project Title:
advertisement
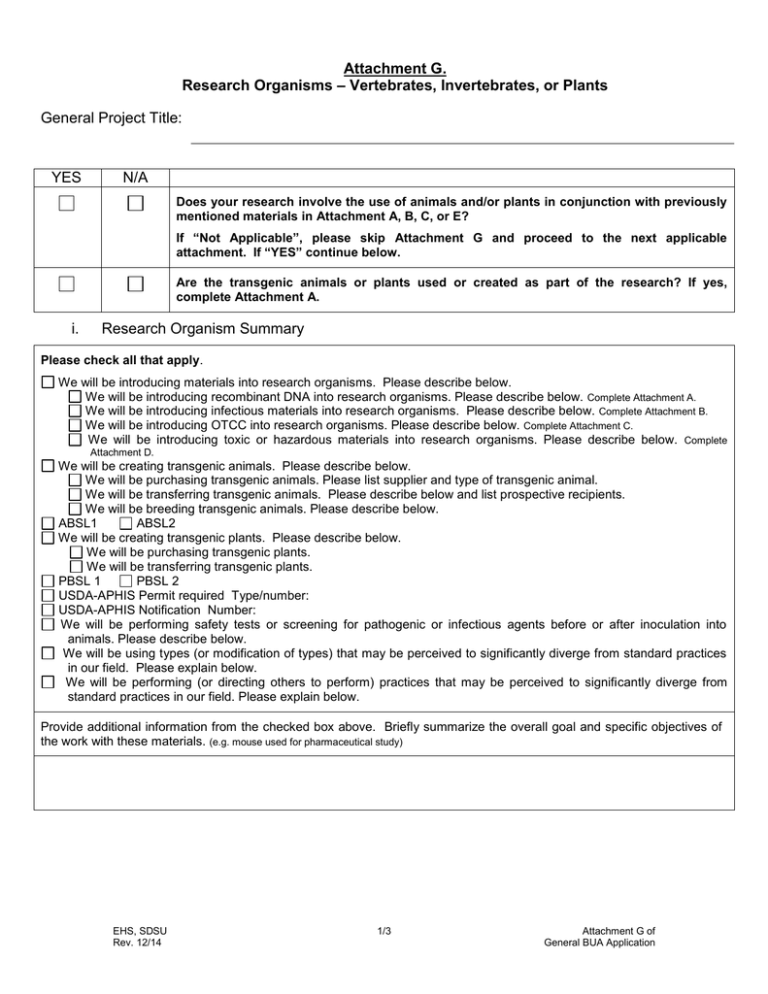
Attachment G. Research Organisms – Vertebrates, Invertebrates, or Plants General Project Title: YES N/A Does your research involve the use of animals and/or plants in conjunction with previously mentioned materials in Attachment A, B, C, or E? If “Not Applicable”, please skip Attachment G and proceed to the next applicable attachment. If “YES” continue below. Are the transgenic animals or plants used or created as part of the research? If yes, complete Attachment A. i. Research Organism Summary Please check all that apply. We will be introducing materials into research organisms. Please describe below. We will be introducing recombinant DNA into research organisms. Please describe below. Complete Attachment A. We will be introducing infectious materials into research organisms. Please describe below. Complete Attachment B. We will be introducing OTCC into research organisms. Please describe below. Complete Attachment C. We will be introducing toxic or hazardous materials into research organisms. Please describe below. Complete Attachment D. We will be creating transgenic animals. Please describe below. We will be purchasing transgenic animals. Please list supplier and type of transgenic animal. We will be transferring transgenic animals. Please describe below and list prospective recipients. We will be breeding transgenic animals. Please describe below. ABSL1 ABSL2 We will be creating transgenic plants. Please describe below. We will be purchasing transgenic plants. We will be transferring transgenic plants. PBSL 1 PBSL 2 USDA-APHIS Permit required Type/number: USDA-APHIS Notification Number: We will be performing safety tests or screening for pathogenic or infectious agents before or after inoculation into animals. Please describe below. We will be using types (or modification of types) that may be perceived to significantly diverge from standard practices in our field. Please explain below. We will be performing (or directing others to perform) practices that may be perceived to significantly diverge from standard practices in our field. Please explain below. Provide additional information from the checked box above. Briefly summarize the overall goal and specific objectives of the work with these materials. (e.g. mouse used for pharmaceutical study) EHS, SDSU Rev. 12/14 1/3 Attachment G of General BUA Application ii. Animal Research Organism If not applicable, check here . If vertebrate species involved, list IACUC protocol ID: Research Organism (Genus, species, strain) Recipient of: rDNA Construct (germ line or somatic?) Vector Microbe Yes No Yes No Yes No iii. Chem -ical Yes No Yes No Yes No Yes No Yes No Yes No Yes No Yes No Yes No Adminis tration Route Transgenic/Genetically Modified Animal Gene Modification Insert Delete/Silence iv. OTCC Gene Name Species of Animal/Strain Max. Vol. Route of Shedding/ Excretion & Interval If not applicable, check here Gene Source or Species Encode for If not applicable, check here Biological Agent or rDNA . Phenotypic results, expected tissue expression ABSL2 Containment Species of Animal/Strain Max. Conc. Admi nister ed . Containment/Work Practice/Locations Prior to procedures # of Animals Infectious Agent During Procedures Post Procedures Duration of Infectivity* *Attach Literature or Reference for the infectious agents’ duration of infectivity for the research organism. v. Plant Research Organism Species of Plant Transgenic Yes Yes Yes vi. No No No If not applicable, check here USDA-APHIS Permit/Notification (Type, Number) Yes Yes Yes Noxious Weed (US or CA) No No No Yes Yes Yes Transgenic/Genetically Modified Plant Method of Reproduction (self, wind pollinator, insect pollinator, human intervention required) No No No If not applicable, check here Recombinants will have special growth requirements: Yes Describe requirements: Recombinants are expected to be more pathogenic: Yes Describe why it is expected to be more pathogenic: No No a. Transformation Method Agrobacterium tumefaciens (all tumorigenic DNA removed) Particle bombardment Vacuum infiltration Other: b. Inserted or Deleted/Silenced Gene Information EHS, SDSU Rev. 12/14 2/3 . Attachment G of General BUA Application . Gene Modification Insert Delete/Silence Gene Name Gene Source or Species Encode for Phenotypic results c. Characterization of Inserted Gene Pharmaceutical Pesticide, microbial pesticide, other plant-incorporated protectant Pesticide resistance Pathogen, pest, or herbicide resistance Selectable marker genes that will not be removed Other: vii. Plant Containment If not applicable, check here Greenhouse PBSL Growth Chamber PBSL (i.e. 1, 2, 3) Pollen Seeds Whole plants Tissue Culture Use PBSL (i.e. 1, 2, 3) (i.e. 1, 2, 3) Yes No Yes No Yes No Yes No Yes No Yes No Yes No Yes No Yes No Motile macrorganisms insects, nematodes Yes No Yes No Yes No Microorganisms Yes No Yes No Yes No viii. Field Release . If not applicable, check here . Describe method for field release and the impact it may have on the normal flora or fauna. ix. Transportation If not applicable, check here . Describe transportation method for transport. Include any methods to prevent release during transport: Indicate transportation locations: x. Methods to Minimize Dissemination and Accidental Release If not applicable, check here Removal of flower and seed heads Harvest prior to sexual maturity Cloaking of flowers to prevent seed and pollen dispersal Use of male sterile lines Keeping distance between infected plants and a susceptible host Choosing season/chronological timing of experiment to prevent cross contamination of susceptible plants Removal of vectors for insect borne transmission Use of plants genetically disabled for survival in the wild Describe environmental impact if accidentally released. Include methods to prevent release if not indicated above: Submission Submit completed Attachment G with General BUA Application to the IBC at Gateway Center 3519D, MC 1933 and an electronic copy to ibc@mail.sdsu.edu. EHS, SDSU Rev. 12/14 3/3 Attachment G of General BUA Application .

