Document 17773203
advertisement

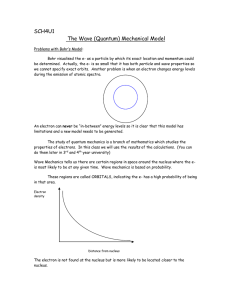
Wave Nature of Matter I. Matter Waves A. Louis de Broglie - 1924 All matter ("particles") also posses wave properties. Louis de Broglie assumes that nature has a basic symmetry. If light travels as a wave but interacts as a particle as claimed by Einstein then particles must also have wave properties! Furthermore, the basic equations must be analogous since all particles are waves and vice-versa. B. Linear Momentum and Wavelength h pM v k To have linear momentum implies that a baseball has a ___wavelength_____________ and _____frequency_________________ . C. Important Concepts and Possible Errors 1. M is the __relativistic___________ _____mass___________ and not the ______rest_____________ ______mass______________ . 2. E pc except for massless particles like photons. You must use the correct energy-linear momentum relationship for problems involving high-speed particles E 2 pc m o c 2 2 2 3. We obtained deBroglie's relationship in an earlier lesson for photons using Einstein's photon hypothesis, E= h. In fact, Louis de Broglie states that his relationship is more fundamental with Einstein's energy relationship being a special case for a zero rest mass particle traveling at speed c. Proof: p h pc hc Since the photon has no rest-mass, we have by relativity that Epc E hc Since light is a wave that travels with the speed of c, we have that c v Substituting into our energy relationship, we have that E h h Example: Determine the wavelength of a 0.25 kg baseball moving at 30 m/s. Solution: Step1 - Do we need to use relativity? Step 2 - Calculate Linear Momentum Step 3 - Use deBroglie relationship to find wavelength II. Davidson-Germer Experiment A. Experimental Setup electrons electron detector Crystal Ni Target B. Result Intensity Angle C. The Key to proving wave phenomena is to find _______interference________. D. Analysis 1. The crystal is acting as a ___diffraction_______________ __grating________ with the maximum in the spectrum due to ___constructive______________ _____interference___________________________. d d Ni Crystal of Spacing d 2. The _______constructive______________ _____interference______________ condition (Bragg Equation) from PHYS2424 is n = = 2 d Sin 3. The atomic spacing, d, could be obtained using _______x-rays___________ ! 4. The linear momentum of the electron could be obtained independently from the electrons kinetic energy. For the 54 eV electron beam, the classical kinetic energy formula is valid: p2 T 2m E. Importance of Experiment - It proved the ____wave______________ _______nature_____________ of matter. Electrons have a _______wavelength_________ ! F. A similar experiment was performed independently later that year by G.P. Thomson (J.J. Thomson's son). Davidson and Thomson won the Nobel Prize for their scattering experiments. III. Bohr Atom and the de Broglie Relationship We can now see the reason for the allowed electron orbits in the Bohr model. These are the only orbits in which the electron waves _______constructively____________ _____interfere__________. Proof: The constructive interference criterion is 2πr n but deBroglie relationship states that h p Thus, we have that nh 2πr p h prn n 2 In Phys1224, we learned that the magnitude of the angular momentum for an object in uniform circular motion is given by L p r sin 90 p r Thus, we have the Bohr relationship that Ln IV. Bohr Correspondence Principle A. There were several problems with old Quantum Mechanics: 1. It predicted spectral lines but stated nothing about the brightness of the lines. 2. Many lines were not seen. This indicated that there were selection rules that determined what lines were present. 3. The system appeared to ba an ad-hoc set of theories with not formal structure. B. One principle designed to provide selection rules is some cases was supplied by Bohr in 1923 and is called the Correspondence Principle. 1. The predictions of Quantum Mechanics for the behavior of a system must be the same as that of classical physics in the limit where quantum numbers become large. 2. A selection rule must hold over the entire range of quantum numbers. These rules can be used to extrapolate QM results to classical physics, but were usually used to determine QM selection rules from classical physics. V. Wilson-Sommerfield Quantization Rules These rules presented in 1916 were an ad-hoc theory extrapolating classical mechanics to provide an overall quantization rule for all periodic coordinates. For any physical system in which the coordinates are periodic functions of time, there exists a quantum condition for each coordinate. These quantum conditions are p q dq n q h where q is one of the generalized coordinates, pq is its canonical momentum, nq is a quantum number which takes on integer values, and means that the integration is taken over one period of the coordinate q. (See Analytical Mechanics – 6th Ed. by Fowles & Cassidy and the Classical Physics 1 Review Module 1 for more about Lagrangian Mech.)