Module 8 – Wave/Particle Duality I. Matter Waves
advertisement
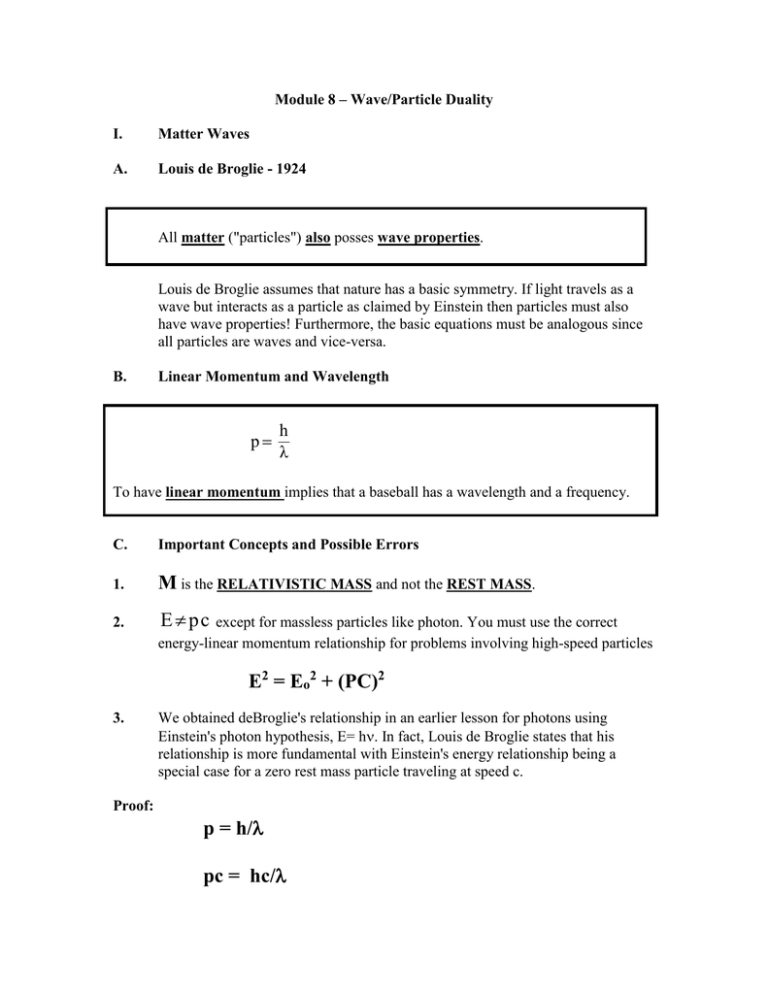
Module 8 – Wave/Particle Duality I. Matter Waves A. Louis de Broglie - 1924 All matter ("particles") also posses wave properties. Louis de Broglie assumes that nature has a basic symmetry. If light travels as a wave but interacts as a particle as claimed by Einstein then particles must also have wave properties! Furthermore, the basic equations must be analogous since all particles are waves and vice-versa. B. Linear Momentum and Wavelength p h λ To have linear momentum implies that a baseball has a wavelength and a frequency. C. Important Concepts and Possible Errors 1. M is the RELATIVISTIC MASS and not the REST MASS. 2. E pc except for massless particles like photon. You must use the correct energy-linear momentum relationship for problems involving high-speed particles E2 = Eo2 + (PC)2 3. We obtained deBroglie's relationship in an earlier lesson for photons using Einstein's photon hypothesis, E= h. In fact, Louis de Broglie states that his relationship is more fundamental with Einstein's energy relationship being a special case for a zero rest mass particle traveling at speed c. Proof: p = h/ pc = hc/ Since the photon has no rest-mass, we have by relativity that E = pc E = hc/ Since light is a wave that travels with the speed of c, we have that c=v= Substituting into our energy relationship, we have that E=h Q.E.D. II. Davidson-Germer Experiment A. Experimental Setup electrons electron detector Crystal Ni Target d B. Result Intensity Angle C. The Key to proving wave phenomena is to find Interference. D. Analysis 1. The crystal is acting as a Diffraction Grating with the maximum in the spectrum due to the Atomic Spacing. d d Ni Crystal of Spacing d 2. The Constructive Interference condition (Bragg Equation) from PHYS2424 is n = = 2 d Sin is the angle between the wave front and the Bragg planes. This will work with any problem. 3. The atomic spacing, d, could be obtained using Xrays which we know are waves! 4. The linear momentum of the electron could be obtained independently from the electrons kinetic energy. For the 54 eV electron beam, the classical kinetic energy formula is valid: K = p2/2m E. Importance of Experiment - It proved the wave property of matter. Electrons have a wavelength! F. A similar experiment was performed independently later that year by G.P. Thomson (J.J. Thomson's son). Davidson and Thomson won the Nobel Prize for their scattering experiments. III. Bohr Atom and the de Broglie Relationship We can now see the reason for the allowed electron orbits in the Bohr model. These are the only orbits in which the electron waves constructively interfere. Proof: The constructive interference criterion is 2r =n but deBroglie relationship states that = h/p Thus, we have that 2 r = nh/p p r = nh/(2) In Phys1224, we learned that the magnitude of the angular momentum for an object in uniform circular motion is given by L = p r Sin(90) = p r Thus, we have the Bohr relationship that L = nh/(2) Q.E.D. IV. Bohr Correspondence Principle A. There were several problems with old Quantum Mechanics: 1. It predicted spectral lines but stated nothing about the brightness of the lines. 2. Many lines were not seen. This indicated that there were selection rules that determined what lines were present. 3. The system appeared to be an ad-hoc set of theories with no formal structure. B. One principle designed to provide selection rules in some cases was supplied by Bohr in 1923 and is called the Correspondence Principle. 1. The predictions of Quantum Mechanics for the behavior of a system must be the same as that of classical physics in the limit where quantum numbers become large. 2. A selection rule must hold over the entire range of quantum numbers. These rules can be used to extrapolate QM results to classical physics, but were usually used to determine QM selection rules from classical physics. V. Wilson-Sommerfield Quantization Rules These rules presented in 1916 were an ad-hoc theory extrapolating classical mechanics to provide an overall quantization rule for all periodic coordinates. For any physical system in which the coordinates are periodic functions of time, there exists a quantum condition for each coordinate. These quantum conditions are p q dq n q h where q is one of the generalized coordinates, pq is its canonical momentum, nq is a quantum number which takes on integer values, and means that the integration is taken over one period of the coordinate q. (See Analytical Mechanics by Fowles for more about Lagrangian Mech.) V. Wave Packets (See Vol. 50 in Mechanical Universe Vide at www.learner.org) A. Philosophical Interpretation 1. Modern physics says that some aspects of an experiment with matter (let us say an electron) can only be explained by the particle representation while others can only be explained with the wave picture. 2. We never see both aspects at the same time. In fact, some physicists believe that the very act of measurement determines reality by creating the result with no deep reality existing prior to the measurement. Other physicists believe that there is deep reality, but that the determinism of classical physics must be abandoned. B. How can a wave describe an object that has a specific location? Although both waves and particles can transfer momentum and energy, they have many other properties that are very different. Particle Definite Location 1+1=2 Definite trajectory (position vector) Wave No Definite Location 1+1 may or may not equal 2 (Interference) No Definite Trajectory A single wave of wavelength, , has a definite momentum, but know specific location. Thus, it can’t represent an object with a definite location. We can form a wave packet by adding many waves with different wavelengths to create an object which is localized at a definite point in space. However, our wave packet know has a range of wavelengths and therefore a range of linear momenta. This tradeoff between the particle aspect and the wave aspect aspect of matter can never be avoided. It is a fundamental limit to our understanding of nature. VI. Fourier Analysis Definition: Any two variable (x, k) are said to be a Fourier Transform Pair if functions of x and k obey the following relationships: 1 F (k ) 2 1 f ( x) 2 f x e ikx dx Fourier Transform of x F k e ikx dk Inverse Fourier Transform 1. Fourier analysis is a technique for representing analytical functions with an infinite number complex exponentials or equivalently sine and cosine functions. 2. Fourier analysis is a useful method of solving many problems in both Physics and Engineering by converting a differential equation in space or time into an algebraic equation in the corresponding momentum or frequency space. In particular, it turns a second order differential equation into a quadratic equation which can be solved by the quadratic formula. The technique is useful provided that either the transform or inverse transform is not more difficult that the original problem. (See any math handbook for a list of functions and their Fourier Transforms; See Mathematical Methods by Physicists by Arfkin for more information on Transforms including Fourier Transforms.) Transform Solve k space problem Simpler problem In k space Difficult problem in x space Inverse Transform Problem Solved 3. The Fourier spectrum of an aperiodic function is continuous. 4. The Fourier spectrum of a periodic function is discrete. 5. There is a fundamental relationship for the product of the uncertainty of any two variables related by a Fourier Transform!! x k 1 2 This fact was known by Classical Physicists when solving problems in classical physics, but it was a mathematical oddity and had no physical reality since an uncertainty in position of a particle might be real, but uncertainty in wave number was meaningless since particles don’t have a wave number. VII. Heisenberg Uncertainty Principle A. Position-Momentum Uncertainty Δx Δp 2 Proof: We start with the uncertainty relationship for the Fourier Transform between position, x, and wave number, k. Δx Δk 1 2 We know multiply both sides by . Δx Δk 2 Δx Δ k 2 Using de Broglie relationship, p k , we have Δx Δp . 2 Q.E.D. A. Energy-Time Uncertainty ΔE Δt 2 Proof: We start with the uncertainty relationship for the Fourier Transform between angular frequency, , and time, t. Δω Δt 1 2 We know multiply both sides by . Δω Δt 2 Δt Δ ω 2 Using Einstein’s relationship, E ω , we have ΔE Δt . 2 Q.E.D. C. General Relationship Heisenberg developed a more general relationship that not only dealt with variables that were connected by Fourier Transforms, but with more generalized quantities that could be represented by operators that do not commute in his version of Quantum Mechanics. We will leave this material for discussion in your Quantum Physics course that you will take later in your undergraduate curriculum. What is important is that you understand how radically our picture of the world has changed. Classical Physics is DETERMINISTIC. In Classical physics it is possible at least theoretically for one to specify exactly both the position and velocity of a particle (i.e. its trajectory). This is what you did in homework problems in Introductory Physics. The Heisenberg uncertainty principle says that it is in fact impossible to do this!! You can not know both the location of an object and how fast it is going!! Every energy level in an atom must have a finite width and not be a specific value like -13.6 eV. When an electron makes a transition from one level to another, we will detect the emitted photon to have a wavelength that is uncertain to some amount E (the line width) regardless of how perfect our detector might be. This uncertainty in energy is related to the time required for the transition (t). An emission with zero uncertainty in energy would require a transition time (t) of infinity (i.e. it would never happen). Thus Quantum Physics is Not Deterministic. It is Probabilistic. We have verified the Heisenberg relationship experimentally over and over again. It is a deep and amazing law of nature that is extremely difficult to comprehend. Bohr and Einstein debated the principle through a series of famous thought experiments in which Bohr always answered Einstein’s challenges. Even today it is the center of philosophical difficulties with new experiments that both amaze and confound the greatest minds in physics. How did we get into this strange mess?? It is the de Broglie hypothesis that matter has wave properties and Einstein’s hypothesis of the particle nature of light which has lead us to this dilemma. We have seen many experiments in which these two hypotheses were confirmed experimentally so we are now stuck with the implications of these experiments.







