Document 17772789
advertisement

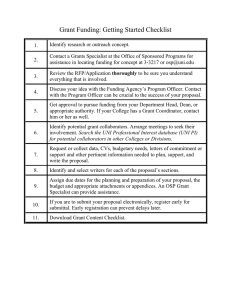
OFFICE OF SPONSORED PROGRAMS OSP Leadership Transition MRAM – October 1, 2015 Lynette Arias – Assistant Vice Provost Research Administration Strategic Initiatives Carol Rhodes – Acting Co-Director, OSP, Compliance and Policy Amanda Snyder – Acting Co-Director, OSP, Operations http://nationaldaycalendar.com/national-research-administrator-day/ OFFICE OF SPONSORED PROGRAMS AVP – Research Administration Strategic Initiatives • • • University wide • Support and advocacy in reducing research administrative burden • Faculty outreach, partnership and support • Affiliation relationship clarity/guidance • University policy development National • Research Administration advocacy • Council on Governmental Relations (COGR) Research & Regulatory Reform Committee • Federal Demonstration Partnership (FDP) Expanded Clearinghouse Workgroup Increased sponsor partnership building / Industry relations International • Global research administration support • Continued support of foreign subrecipient partnerships OFFICE OF SPONSORED PROGRAMS Acting Co-Directors • Compliance & Policy – Carol • Operations – Amanda OFFICE OF SPONSORED PROGRAMS Compliance & Policy • • • • • • • • • GIM development/maintenance F&A waivers/F&A questions GIM 19 waivers Regulatory compliance process development Campus workgroup and initiatives related to risk management & assessment, policy development FOIA requests/Public Records requests Master Agreement Review & Signature Audit Assistance (with IA, when sponsored programs) External groups/meetings including MRAM, RAPIT, other Process Improvement groups OFFICE OF SPONSORED PROGRAMS Operations • Oversight of day-to-day operations • Authorized Institutional Official • Campus Outreach Lead • Process Coordination with GCA and Procurement • External groups/meetings including MRAM, RAPIT, other Process Improvement groups OFFICE OF SPONSORED PROGRAMS Thank you! OFFICE OF SPONSORED PROGRAMS OFFICE OF SPONSORED PROGRAMS Subawards Update Amanda Snyder Acting Co-Director, OSP http://nationaldaycalendar.com/national-research-administrator-day/ Use of Ariba now required for subawards • As Procurement has communicated, PAS is closed for requisitions as of today, 10/1/2015 • OSP must receive new and modification requests via Ariba • Transition of purchase orders from PAS to Ariba continues • If you need to modify a purchase order that is not yet in Ariba, please send an email to aribasub@uw.edu • PO will be transitioned to Ariba • You will then be able to request modifications and “receive” invoices in Ariba OFFICE OF SPONSORED PROGRAMS New tool for visibility • Subaward data is now available via the Enterprise Data Warehouse (EDW) https://biportal.uw.edu/Report/Details/ResearchSubawards • On the Business Intelligence (B.I.) Portal • • • • Works the same as award & proposal reports Searchable report Updated nightly, so essentially “real time” data Need the ASTRA permission to gain access (more info at http://www.washington.edu/itconnect/work/data/use-data/get-access/request-access/) • Note: the “Assigned To” field is currently missing from report, will be available 10/9 OFFICE OF SPONSORED PROGRAMS OFFICE OF SPONSORED PROGRAMS Intake of Subaward Requests for OSP Business process reengineering effort is in the works for the OSP Subawards Intake process • Actively working through details with various campus partners • Vision: leverage the data we have in SAGE/SPAERC so that system supports both campus and OSP needs for subs • Ideally, an intake process similar to the eGC1 • Automated assignment • 2-way collaboration • Visibility to your requests in progress OFFICE OF SPONSORED PROGRAMS Intake of Subaward Requests for OSP (Cont.) • Will enable parallel work on subaward agreement lifecycle and Ariba Contract Request process. • Will clarify roles and responsibilities between financial and contractual • Ariba will still be required for the financial portion of subaward actions • We may need to pursue an interim, “lower tech” solution in the interim. Planning is underway. OFFICE OF SPONSORED PROGRAMS New Subaward Templates in the Works • The Federal Demonstration Project (FDP) forms have been updated and we plan to begin using them later this month • Updates for the Uniform Guidance • Still awaiting agency-specific T & C’s (Attachment 2) from all federal agencies except NSF & NIH • Update to Attachment 4, Reporting Requirements, is going to require additional info from campus OFFICE OF SPONSORED PROGRAMS OFFICE OF SPONSORED PROGRAMS Advance Budget Numbers and Industry Sponsored Clinical Trials Carol Rhodes Acting Co-Director, OSP MRAM October 2015 Issue Being Addressed Inability to get an advance budget number for an anticipated industrysponsored clinical trial Symptoms: • Increased salary transfer activity at the beginning of the trial • Errors in the transfer of expenditures from dept budget number to clinical trial budget number • Inconvenience of housing start-up costs on one or more department budget numbers • Lack of information/awareness of roles and responsibilities with respect to pre-final contract spending, if not running through sanctioned UW advance budget number process New Process as of October 5th Advance Budget Number Eligibility for Industry Sponsored Clinical Trials Department routes eGC1 to OSP OSP reviews content of eGC1 and approves OSP creates a Funding Action (FA) while negotiating the Clinical Trial Agreement Department may use the Advance Budget Number (BN) Eligibility Tool to request an Advance BN This is routed directly to a Program Coordinator Team in OSP It is reviewed and if eligible, OSP removes the block on advance budget number eligibility Department may then enter SAGE and send in the details for budget number set-up to GCA Advance Budget Number Review The same as for any sponsored program, including the following: • If no pre-award spending authority, is department willing to incur the risk and supply an alternate department budget number? • Will human subjects research expenditures be charged to the advance budget number, and if so, is there IRB approval (WIRB approval, in this case)? • Have Investigators disclosed their SFI and has it been reviewed? Can I get a Research Study Account (“Triple R”) activated with an advance budget number? No. Here is why: o Triple R account allows for billing of clinical services. o Clinical services should not occur without a fully-executed clinical trial agreement in place. o If you supply an advance budget number to CRBB to activate your Triple R account, CRBB will notify you that the advance budget number is not allowed for this request and point you back to OSP policy. Questions? • Advance Budget Number Primer and Eligibility Tool • Clinical Trial definition and other resources related to budgeting, IRB approval, FCOI, clinical trial lifecycle (Roles/Responsibilities) Thank you! UPCOMING COURSES IN RESEARCH ADMINISTRATION Oct 05 GCA 202 – Direct Billing of F&A Type Costs Oct 07 MAA 101 – Introduction to Faculty Effort Certification Oct 08 OSP 101 – Introduction to Research Administration Oct 14 MAA 102 – Electronic Faculty Effort Certification (eFECS) for FEC Coordinators Oct 22 GCA 100 – Post-Award Financial Administration: Processes, Offices and Best Practices Oct 28 MAA 100 – Introduction to Grant and Contract Certification (GCCR) Oct 28 GCA 201 – Salary and Cost Transfers and Compliance Nov 11 MAA 220 – Managing Faculty Effort Nov 09 MAA 215 – Faculty Effort and Cost Share: Calculate it Right! CORE is a collaborative effort to provide free, high quality, cohesive training on research administration and compliance to UW researchers and research administrators. To see a full list of courses and to register, visit https://uwresearch.gosignmeup.com/public/course/browse CORE CAMPUS SURVEY RESULTS We received 172 responses to the CORE survey MAJOR THEMES Requests for more advanced course work More frequent scheduling of introductory courses More examples/scenarios to work through in class Access to Online Resources THANK YOU!