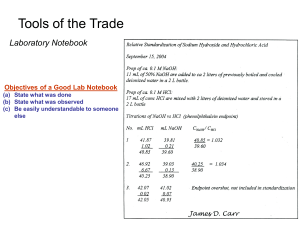
Tools of the Trade Laboratory Notebook Objectives of a Good Lab Notebook
advertisement

Tools of the Trade Laboratory Notebook Objectives of a Good Lab Notebook (a) State what was done (b) State what was observed (c) Be easily understandable to someone else Tools of the Trade Laboratory Notebook Bad Laboratory Practice (A Recent Legal Case) Medichem Pharmaceuticals v. Rolabo Pharmaceuticals Two Patents describe a method for making the antihistamine drug Loratidine (Claritin) - US sales of $2.7 billion - the two patents are essentially identical - Medichem sued to invalidate Rolabo patent and claimed priority - Medichem had to prove it used the method to make loratidine before Rolabo did A co-inventor’s lab notebook was a primary piece of evidence to support Medichem’s claim - documented analysis of a sample claimed to be made using the patented method - NMR spectral data confirmed the production of loratidine The evidence was not enough to support Medichem's claim of reduction to practice - NMR data do not show the process by which loratidine was made - lab books were not witnessed Rolabo Pharmaceuticals won the case (and the rights to make Loratidine) because of problems with a Lab Notebook!! Nature Reviews Drug Discovery (2006) 5, 180 Tools of the Trade Weight Measurements 1.) Analytical Balance (principal of operation): (i) sample on balance pushes the pan down with a force equal to m x g M is mass of object g is acceleration of gravity (ii) balance pan with equal and opposing mass Mechanical – standard masses Electronic – opposing electromagnetic force (iii) tare –mass of empty vessel (pan) l1 l2 Double-pan balance m1 m2 m m m1l1 = m2l2 (a) balance beam suspended on a sharp knife edge (b) Standard weights are added to the second pan to balance sample weight (c) Weight of sample is equal to the total weight of standards Tools of the Trade Weight Measurements Single-pan balance (a) (b) (c) (d) balance beam suspended on a sharp knife edge Sample pan is balanced by counterweights on right Knob adjusted to remove weights from a bar above the pan Pan is moved back to its original position and the removed weights equals the mass of the sample. Tools of the Trade Weight Measurements Electronic balance (a) Uses electromagnetic force to return the pan to original position (b) Electric current required to generate the force is proportional to sample mass Determines amount of deflection of pan due to mass of sample Increase in current causes magnetic field that raises pan Tools of the Trade Weight Measurements 2.) Methods of Weighing: (i) Basic operational rules Chemicals should never be placed directly on the weighing pan - corrode and damage the pan may affect accuracy - not able to recover all of the sample Balance should be in arrested position when load/unload pan Half-arrested position when dialing weights - dull knife edge and decrease balance sensitivity accuracy (ii) Weight by difference: Useful for samples that change weight upon exposure to the atmosphere - hygroscopic samples (readily absorb water from the air) Weight of sample = ( weight of sample + weight of container) – weight of container (iii) Taring: Done on many modern electronic balances Container is set on balance before sample is added Container’s weight is set automatically to read “0” Tools of the Trade Weight Measurements 3.) Errors in Weighing: Sources (i) Any factor that will change the apparent mass of the sample Dirty or moist sample container - also may contaminate sample Sample not at room temperature - avoid convection air currents (push/lift pan) Adsorption of water, etc. from air by sample Vibrations or wind currents around balance Non-level balance Tolerance (mg) Tolerance (mg) Grams Class 1 Class 2 Milligrams Class 1 Class 2 500 1.2 2.5 500 0.010 0.025 200 0.5 1.0 200 0.010 0.025 100 0.25 0.50 100 0.010 0.025 50 0.12 0.25 50 0.010 0.014 20 0.074 0.10 20 0.010 0.014 10 0.050 0.074 10 0.010 0.014 5 0.034 0.054 5 0.010 0.014 2 0.034 0.054 2 0.010 0.014 1 0.034 0.054 1 0.010 0.014 Office dust Tools of the Trade Weight Measurements 3.) Errors in Weighing: Sources (i) Any factor that will change the apparent mass of the sample Buoyancy errors – failure to correct for weight difference due to displacement of air by the sample. balsa Different displacement of ice and balsa wood in water ice Correction for buoyancy to give true mass of sample m' ( 1 m (1 da ) dw da ) d m = true mass of sample m’ = mass read from balance d = density of sample da = density of air (0.0012 g/ml at 1 atm & 25oC) dw = density of calibration weights (~ 8.0 g/ml) Tools of the Trade Weight Measurements 3.) Errors in Weighing: Sources Example: The densities (g/ml) of several substances are: acetic acid 1.05 lithium 0.53 lead 11.4 CCl4 1.59 mercury 13.5 iridium 22.5 Sulfur 2.07 PbO2 9.4 From the following figure: predict which substance will have the smallest percentage buoyancy correction and which will have the greatest. Tools of the Trade Weight Measurements 3.) Errors in Weighing: Sources (i) Any factor that will change the apparent mass of the sample Density of air changes with temperature and pressure To get da under non standard conditions d a 0.46468 ( B 0.3783V ) T B = Barometer pressure (torr) V = vapor pressure of water in the air (torr) T = air temperature (K) Tools of the Trade Volume Measurements 1.) Burets (i) Purpose: used to deliver multiple aliquots of a liquid in known volumes Buret volume (ml) Smallest graduation (ml) Tolerance (mL) 5 0.01 ± 0.01 10 0.05 or 0.02 ± 0.02 25 0.1 ± 0.03 50 0.1 ± 0.05 100 0.2 ± 0.10 (ii) Correct use of buret Read buret at the bottom of a concave meniscus Meniscus at 9.68 mL Tools of the Trade Volume Measurements 1.) Burets (iii) always read the buret at the same eyelevel as the liquid Avoids parallax errors eyelevel View from above 15.46 mL 15.31 mL 1% error (iv) Consistently read all levels versus a given position on the nearest mark Tools of the Trade Volume Measurements 1.) Burets (v) Estimate the buret reading to the nearest 1/10 of a division (vi) expel all air bubbles from the stopcock prior to use (vii) rinse the buret with a solution 2-3x before filling the buret for a titration (viii) Near the end of a titration, volume of 1 drop or less per delivery should be used with the buret. Tools of the Trade Volume Measurements 2.) Volumetric Flasks (i) Purpose: used to prepare a solution of a single known volume (ii) Types of volumetric flasks Flask Capacity (mL) Tolerance (mL) 1 ± 0.02 2 ± 0.02 5 ± 0.02 10 ± 0.02 25 ± 0.03 50 ± 0.05 100 ± 0.08 200 ± 0.10 250 ± 0.12 500 ± 0.20 1000 ± 0.30 2000 ± 0.50 Tools of the Trade Volume Measurements 2.) Volumetric Flasks (iii) Correct use of volumetric flask Add reagent or solution to flask and dissolve in volume of solvent less than the total capacity of the flask Slowly add more solvent until the meniscus bottom is level with the calibration line. stopper Stopper the flask and mix solution by inversion (40 or more times) (for later use) Remix by inverting the flask if the solution has been sitting unused for more than several hours Glass adsorbs trace amount of chemicalsclean using acid wash - adhere to surface Tools of the Trade Volume Measurements 3.) Pipets and Syringes (i) Use to deliver a given volume of liquid (ii) Types of pipets Transfer pipet Transfer Pipet - similar to volumetric flask Volume (mL) Tolerance (mL) - transfers a single volume fill to calibration mark - last drop does not drain out of the pipet do not blow out 0.5 ±0.006 - more accurate than measuring pipet 1 ±0.006 2 ±0.006 3 ±0.01 4 ±0.01 5 ±0.01 10 ±0.02 15 ±0.03 20 ±0.03 25 ±0.03 50 ±0.05 100 ±0.08 Measuring pipet - calibrated similar to buret - use to delivery a variable volume Tools of the Trade Volume Measurements 3.) Pipets and Syringes (ii) Types of pipets Micropipet - deliver volumes of 1 to 1000 mL (fixed & variable) - uses disposable polypropylene tip - stable in most aqueous and organic solvents (not chloroform) - need periodic calibration 10% of pipet volume 100% of pipet volume Accuracy (%) Precision (%) Accuracy (%) Precision (%) 0.2-2 ±8 ±4 ±1.2 ±0.6 1-10 ±2.5 ±1.2 ±0.8 ±0.4 2.5-25 ±4.5 ±1.5 ±0.8 ±0.2 10-100 ±1.8 ±0.7 ±0.6 ±0.15 30-300 ±1.2 ±0.4 ±0.4 ±0.15 100-1000 ±1.6 ±0.5 ±0.3 ±0.12 10 ±0.88 ±0.4 25 ±0.88 ±0.3 100 ±0.5 ±0.2 500 ±0.4 ±0.18 1000 ±0.33 ±0.12 Pipet volume (mL) Adjustable pipets Fixed pipets Disposable tip Tools of the Trade Volume Measurements 3.) Pipets and Syringes (ii) Types of pipets Syringes - deliver volumes of 1 to 500 mL - accuracy & precision ~0.5-1% - steel needle permits piercing stopper to transfer liquid under controlled atmosphere > attacked by strong acid and contaminate solution with iron (iii) Correct use of pipets and syringes Use a bulb, never your mouth, for drawing solutions into pipets. Rinse pipets and syringes before using - remove bubbles Tools of the Trade Filtration 1.) Mechanical separation of a liquid from the undissolved particles floating in it. 2.) Purpose: used in gravimetric analysis for analysis of a substance by the mass of a precipitate it produces (i) Solid collected in paper or fritted-glass filters 3.) Process: (i) pour slurry of precipitate down a glass rod to prevent splattering. (ii) dislodge solid from beaker/rod with rubber policeman (iii) use wash liquid (squirt bottle) to transfer particles to filter paper (iv) dry sample Rubber policeman Tools of the Trade Drying 1.) Purpose: (i) to remove moisture from reagents or samples (ii) to convert sample to a more readily analyzable form 2.) Oven Drying: commonly used for reagent or sample preparation (i) (ii) Typically 110 oC for H2O removal Use loose covers to prevent contamination from dust 3.) Dessicator: used to cool and store reagent or sample over long periods of time. (i) Contains a drying agent to absorb water from the atmosphere (ii) Airtight seal Experimental Error & Data Handling Introduction 1.) There is error or uncertainty associated with every measurement. (i) except simple counting 2.) To evaluate the validity of a measurement, it is necessary to evaluate its error or uncertainty You can read the name of the boat on the left picture, which is lost in the right picture. Can you read the tire manufacturer? Same Picture Different Levels of Resolution Experimental Error & Data Handling Significant Figures 1.) Definition: The minimum number of digits needed to write a given value (in scientific notation) without loss of accuracy. (i) Examples: 142.7 = 1.427 x 102 Both numbers have 4 significant figures 0.006302 = 6.302 x10-3 Zeros are simple place holders 2.) Zeros are counted as significant figures only if: (i) occur between other digits in the number 9502.7 or 0.9907 Both zeros are significant figures (ii) occur at the end of number and to the right of the decimal point 177.930 zero is a significant figure Experimental Error & Data Handling Significant Figures 3.) The last significant figure in any number is the first digit with any uncertainty (i) the minimum uncertainty is ± 1 unit in the last significant figure (ii) if the uncertainty in the last significant figure is ≥ 10 units, then one less significant figure should be used. (iii) Example: 9.34 ± 0.02 3 significant figures 6.52 ± 0.12 should be 6.5 ± 0.1 2 significant figures But 4.) Whenever taking a reading from an instrument, apparatus, graph, etc. always estimate the result to the nearest tenth of a division (i) avoids losing any significant figures in the reading process 7.45 cm Experimental Error & Data Handling Significant Figures 5.) Addition and Subtraction (i) use the following procedure: Express all numbers using the same exponent Align all numbers with respect to the decimal point 1.25 x 105 2.48 x 104 + 1.235 x 104 12.5 x 104 2.48 x 104 + 1.235 x 104 Add or subtract using all given digits Round off the answer so that it has the same number of digits to the right of the decimal as the number with the fewest decimal places 12.5 2.48 + 1.235 16.215 x x x x 104 104 104 104 1 decimal point = 16.2 x 104 Experimental Error & Data Handling Significant Figures 5.) Addition and Subtraction (i) use the following procedure: Round off the answer to the nearest digit in the least significant figure. Consider all digits beyond the least significant figure when rounding. If a number is exactly half-way between two digits, round to the nearest even digit. - minimizes round-off errors Examples: 3 sig. fig.: 12.534 12.5 4 sig. fig.: 11.126 11.13 4 sig. fig.: 101.250 101.2 3 sig. fig. 93.350 93.4 Experimental Error & Data Handling Significant Figures 6.) Multiplication and Division (i) use the following procedure: Express the answers in the same number of significant figures as the number of digits in the number used in the calculation which had the fewest significant figures. Examples: 3.261 x 10-5 x 1.78 5.80 x 10-5 34.60 ) 2.4287 14.05 3 significant figures 4 significant figures Experimental Error & Data Handling Significant Figures 7.) Logarithms and Antilogarithms (i) the logarithm of a number “a” is the value “b”, where: a = 10b or Log(a) = b (ii) example: The logarithm of 100 is 2, since: 100 = 102 (iii) The antilogarithm of “b” is “a” a = 10b (iv) the logarithm of “a” is expressed in two parts Log(339) = 2.530 character mantissa Experimental Error & Data Handling Significant Figures 7.) Logarithms and Antilogarithms (v) when taking the logarithm of a number, the number of significant figures in the resulting mantissa should be the same as the total number of significant figures in the original number “a” (vi) Example: Log(5.403 x 10-8) = -7.2674 4 sig. fig. 4 sig. fig. (vii) when taking the antilogarithm of a number, the number of significant figures in the result should be the same as the total number of significant figures in the mantissa of the original logarithm “b” (viii) Example: Antilog(-3.42) = 3.8 x 10-4 2 sig. fig. 2 sig. fig. Experimental Error & Data Handling Significant Figures 8.) Graphs (i) use graph paper with enough rulings to accurately graph the results (ii) plan the graph coordinates so that the data is spread over as much of the graph as possible (iii) in reading graphs, estimate values to the nearest 1/10 of a division on the graph Experimental Error & Data Handling Significant Figures 8.) Graphs (ii) plan the graph coordinates so that the data is spread over as much of the graph as possible (iii) in reading graphs, estimate values to the nearest 1/10 of a division on the graph Experimental Error & Data Handling Errors 1.) Systematic (or Determinate) Error (i) An error caused consistently in all results due to inappropriate methods or experimental techniques. (ii) Results in all measurements exhibiting a definite difference from the true value. (iii) This type of error can, in principal, be discovered and corrected. Buret incorrectly calibrated Experimental Error & Data Handling Errors 2.) Random (or Indeterminate) Error (i) An error caused by random variations in the measurement of a physical quantity. (ii) Results in a scatter of results centered on the true value for repeated measurements on a single sample. (iii) This type of error is always present and can never be totally eliminated True value Random Error Systematic Error Experimental Error & Data Handling Errors 3.) Accuracy and Precision (i) Accuracy: refers to how close an answer is to the “true” value Generally, don’t know “true” value Accuracy is related to systematic error (ii) Precision: refers to how the results of a single measurement compares from one trial to the next Reproducibility Precision is related to random error Low accuracy, low precision High accuracy, low precision Low accuracy, high precision High accuracy, high precision Experimental Error & Data Handling Errors 4.) Absolute and Relative Uncertainty (i) Both measures of the precision associated with a given measurement. (ii) Absolute uncertainty: margin of uncertainty associated with a measurement (iii) Example: If a buret is calibrated to read within ± 0.02 mL, the absolute uncertainty for measuring 12.35 mL is: Absolute Uncertainty = 12.35 ± 0.02 mL (iv) Relative uncertainty: compares the size of the absolute uncertainty with the size of its associated measurement Absolute Uncertaint y Relative Uncertaint y Measured Value (v) Example: For a buret reading of 12.35 ± 0.02 mL, the relative uncertainty is: (Make sure units cancel) 0.02 mL Relative Uncertaint y(%) (100) 0.16% 0.2% 12.35 mL 1 sig. fig. Experimental Error & Data Handling Errors 5.) Propagation of Uncertainty (i) The absolute or relative uncertainty of a calculated result can be estimated using the absolute or relative uncertainties of the values used to obtain that result. (ii) Addition and Subtraction The absolute uncertainty of a number calculated by addition or subtraction is obtained by using the absolute uncertainties of numbers used in the calculations as follows: Abs . Uncert .Answer Abs . Uncert . Abs . Uncert .Answer 2 value 2 2 value1 Value 1.76 + 1.89 - 0.59 Answer: 3.06 Example: Abs . Uncert . Abs. Uncert. (± 0.03) (± 0.02) (± 0.02) 0.032 0.022 0.022 0.04 Experimental Error & Data Handling Errors 5.) Propagation of Uncertainty (iii) Once the absolute uncertainty of the answer has been determined, its relative uncertainty can also be calculated, as described previously. Example (using the previous example): 0.04 Re l . Uncert .(%) ( 100 ) 1.3% 1% 3.06 1 sig. fig. Note: To avoid round-off error, keep one digit beyond the last significant figure in all calculations. - drop only when the final answer is obtained Round-off errors Experimental Error & Data Handling Errors 5.) Propagation of Uncertainty (i) Multiplication and Division The relative uncertainties are used for all numbers in the calculation Re l . Uncert .Answer Re l . Uncert . Re l . Uncert . 2 value1 2 value 2 Example: 1.76 0.03 1.89 0.02 5.64 0.59 0.02 Re l . Uncert . 3 sig. fig. 0.03 ( 100 ), 0.02 ( 100 ) , 0.02 ( 100 ) 1.76 1.89 0.59 Re l . Uncert . 1.7%, 1.1% , 3.4% Re l . Uncert .Answer 1.7 2 1.12 3.4 2 4.0% 4% 1 sig. fig. Experimental Error & Data Handling Errors 5.) Propagation of Uncertainty (ii) Once the relative uncertainty of the answer has been obtained, the absolute uncertainty can also be calculated: Relative Uncertaint y(%) Absolute Uncertaint y ( 100 ) Calculated Value Rearrange: Absolute Uncertaint y Relative Uncertaint y(%) (calculate d value) ( 100 ) (iii) Example (using the previous example): 4.0% Absolute Uncertaint y ( 5.64 ) 0.23 0.2 100 1 sig. fig. Experimental Error & Data Handling Errors 5.) Propagation of Uncertainty (iv) For calculations involving Both additions/subtractions and multiplication/divisions: Treat calculation as a series of individual steps Calculate the answer and its uncertainty for each step Use the answers and its uncertainty for the next calculation, etc. Continue until the final result is obtained (v) Example: 1.76 0.03 0.59 0.02 0.619 ? 1.89 0.02 3 sig. fig. First operation: differences in brackets 1.76 0.03 0.59 0.02 1.17 0.036 0.036 0.032 0.02 2 3 sig. fig. 1 sig. fig., but carry two sig. fig. through calculation Experimental Error & Data Handling Errors 5.) Propagation of Uncertainty (v) Example: Second operation: Division 1.17 0.036 1.17 3.1% 0.61% 3% 1.89 0.02 1.89 1.1% 3.3% 3% 3.1% 1.1% 2 Convert to relative uncertainty 3 sig. fig. 2 1 sig. fig. Experimental Error & Data Handling Errors 5.) Propagation of Uncertainty (vi) Uncertainty of a result should be consistent with the number of significant figures used to express the result. (vii) Example: 1.019 (±0.002) 28.42 (±0.05) Result & uncertainty match in decimal place But: 12.532 (±0.064) too many significant figures 12.53 (±0.06) reduce to 1 sig. fig. in uncertainty same reduction in results The first digit in the answer with any uncertainty associated with it should be the last significant figure in the number. Experimental Error & Data Handling Errors 5.) Common Mistake (vi) Number of Significant Figures is Not the number shown on your calculator. Not 10 sig. fig. 23.97 2.596966414 9.23 Experimental Error & Data Handling Errors Example Find the absolute and percent relative uncertainty and express the answer with a reasonable number of significant figures: [4.97 ± 0.05 – 1.86 ± 0.01]/21.1 ± 0.2 =