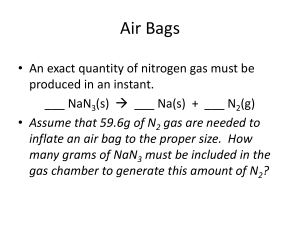PRACTICE PROBLEMS NAME ____________________________
advertisement

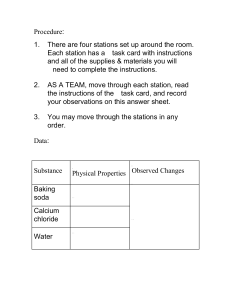
PRACTICE PROBLEMS NAME ____________________________ 1) Washing Soda is used to prepare hard water for washing laundry. Its formula is Na2CO3XH2O. When a 2.558 gram sample of washing soda is heated, all the water of hydration is lost, leaving 0.948 grams of the dehydrated salt. What is the value of X? 2) A poisonous compound being produced by your factory has the following composition: 26.56% K 35.41% Cr 38.03% O. Determine the empirical formula of this compound. 3) An unknown alcohol undergoes combustion analysis according to the following equation: CxHyOz + O2 --- CO2 + H2O. When 2.38 mg of the alcohol is burned, it produces 3.48 mg CO2, and 1.43mg of H2O. The actual molar mass of the alcohol is determined to be 90.0 g/mol from a different experiment. Determine the Empirical Formula and the Molecular Formula of the alcohol.