Document 17624020
advertisement

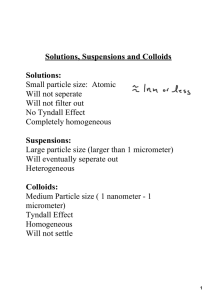
solute – the part of a solution that is being dissolved (usually the lesser amount) solvent – the part of a solution that dissolves the solute (usually the greater amount) solution = solute + solvent Solute Solvent Solution Si Al metal alloy salt water salt water CO2 water carbonated water O N air a measure of the amount of solute that is dissolved in a given quantity of solvent Molarity (M) which is moles/volume volume must be in liters often use square brackets to mean concentration [HCl] = 1.0 M The number of moles of solute dissolved in liters of solution Molarity = moles solute liters of solution 1.0 L of water was used to make 1.0 L of solution. Notice the water left over. 1.0L of water would be too much. top off to 1 liter What is the concentration of 2 moles of HCl in 4 L? 2 mol / 4 L = 0.5 M Step 1: Convert 5 g of NiCl2• 6H2O to moles 1 mol 5.00 g • = 0.0210 mol 237.7 g Step 2: Calculate Molarity 0.0210 mol = 0.0841 M 0.250 L 1.0 L = 1000 mL .25 L = 250 mL What mass of oxalic acid, H2C2O4, is required to make 250 mL of a 0.0500 M solution? M = moles/V same as moles = M•V Step 1: Change mL to L. 250 mL/1000 = 0.250 L Step 2: Calculate. moles = (0.0500 M) (0.250 L) = 0.0125 moles H2C2O4 Step 3: Convert moles to grams. (0.0125 mol H2C2O4) (90.00 g) = 1 mol H2C2O4 1.13 g How many grams of NaOH are required to prepare 400 mL of 3.0 M NaOH solution? 1) 12 g 2) 48 g 3) 300 g