Document 17624018
advertisement

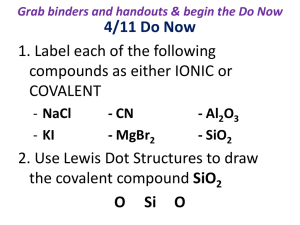
Reactants: Zn + I2 Product: ZnI2 used to convey as much info. as possible about what happens in a chemical reaction Word Equations write out what chemicals are reacting hydrogen peroxide → water + oxygen hydrogen + oxygen → water Chemical Equations show the chemical formulas of the chemicals reacting H2O2 (aq) → H2O(l) + O2(g) H2(g) + O2(g) → H2O (l) (s) = solid, (l) = liquid, (g) = gas, (aq) = aqueous solution (dissolved in water) the above are often referred to as skeletal equations because they are not mathematically balanced chemical equations show the conversion of reactants (the molecules shown on the left of the arrow) into products (the molecules shown on the right of the arrow). a (+) sign separates molecules on the same side the arrow is read as “yields” example C + O2 CO2 this reads “carbon plus oxygen react to yield carbon dioxide” a catalyst is a substance that speeds up the reaction but is not used up in the reaction MnO4 H2(g) + O2(g) H2O (l) this is written small over the yield arrow each side of the equation must have the same number of atoms of each element bicycle example frame + wheel + handlebar + pedal → bike frame + 2 wheel + handlebar + 2 pedal → bike tricycle example frame + wheels + handlebar + tire → tricycle frame + 3 wheel + handlebar + 3 tire → tricycle when balancing a chemical reaction you may add coefficients in front of the compounds to balance the reaction coefficients represent the number of atoms (or moles) of that compound sometimes easier if you balance O and H at the end you may NOT change the subscripts changing the subscripts changes the compound ex: H2O (water) cannot be changed into H2O2 (hydrogen peroxide) in order to help you balance the equation H2(g) + O2(g) → H2O (l) subscripts tell you how many atoms of a particular element are in a compound. coefficients tells you about the quantity, or number, of molecules of the compound this is NOT balanced 1. A solution of sodium iodide is added to a solution of potassium nitrate yields a potassium iodide precipitate and a sodium nitrate solution. NaI (aq) + KNO3 (aq) KI (s) + NaNO3 (aq) already balanced 2. Magnesium metal burns in oxygen gas with a bright white light to make a white powder called magnesium oxide. Mg (s) + O2 (g) MgO (s) + heat 2Mg (s) + O2 (g) 2MgO (s) + heat 3. Gaseous hydrogen (dihydride) and gaseous oxygen (dioxide) react explosively to form water vapor. H2 (g) + O2 (g) H2O (g) + heat 2H2(g) + O2(g) 2H2O (g) + heat ___ 2 Al(s) + ___ 3 Br2(l) ---> ___ Al2Br6(s) ? Al(s) + O2(g) Al2O3(s) 4Al(s) + 3O2(g) 2Al2O3(s) NaCl (aq) + AgNO3 (aq) AgCl (s) + NaNO3 (aq) balanced Na2SiO3 + HF H2SiF6 + NaF + H2O Na2SiO3 + 8HF H2SiF6 + 2NaF + 3H2O