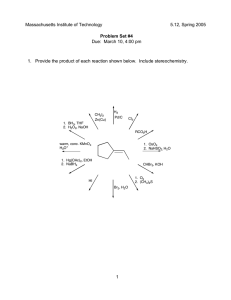
Chemical vs. Physical Properties & Reactions Slides
advertisement
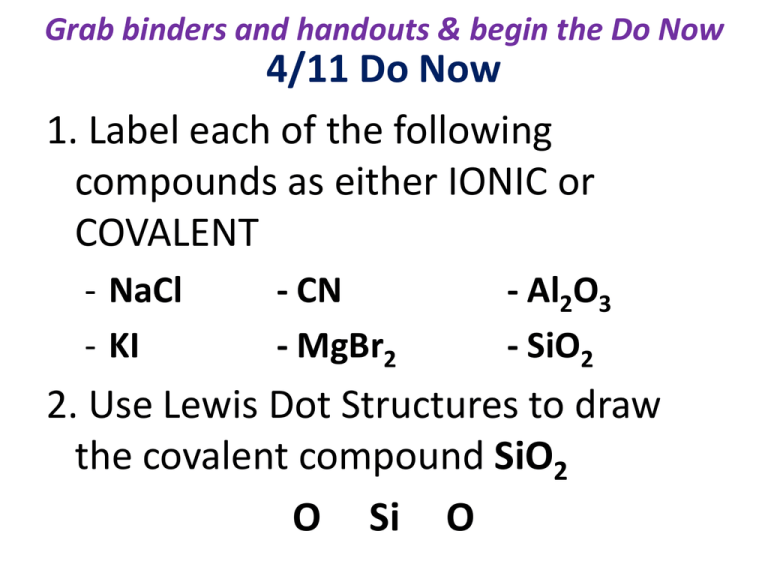
Grab binders and handouts & begin the Do Now 4/11 Do Now 1. Label each of the following compounds as either IONIC or COVALENT - NaCl - KI - CN - MgBr2 - Al2O3 - SiO2 2. Use Lewis Dot Structures to draw the covalent compound SiO2 O Si O While watching… • Set up the following T-chart: What happened? How do I know that happened? Reactions are EVERYWHERE! Jot down your thoughts… • What happens during and after you eat a meal? • How do you know that has happened? What is your evidence? Chemical & Physical Properties & Reactions: Key Point # 1 Physical Properties Chemical Properties -Anything you can SEE, -Reactivity FEEL, SMELL, MEASURE H2O2 reacts w/catalase Odor H2O does not Mass Volume/Shape -Magnetic video Texture Melt, Boil, Freeze Temps. - Acidity State of matter pH scale Color Key Point # 2 Physical Rxns Chemical Rxns - Only 1 compound involved - Something new is created (start and end w/ same compound) - Irreversible - Reversible - Changes state of matter - No bonds breaking & reforming H2O(l) H2O(g) - Break & reform bonds H2O2 H2O + O2 INDICATORS: - Create something new - gas evolution - Temp change - Color change - Light emission Mini-lab Stations - MUST wear goggles & gloves - 6 min at each station - Make sure to CLEAN UP before moving to the next station (even if not completely finished with write up for that lab station) - Today’s Exit Ticket: complete write ups for each lab station and check off with Ms. Herndon Exit Ticket 2 Directions: You have 10 minutes to complete the Exit Ticket to the best of your ability. You MAY NOT use notes, but you may use a Periodic Table if needed. When finished submit to the class bucket.