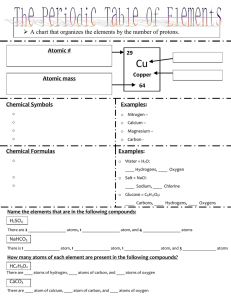
Cu + A chart that organizes the elements by the number...
advertisement

A chart that organizes the elements by the number of protons. Atomic # = number of protons = number of electrons = number of protonsAtomic = number mass of electrons = number of protons 29 Cu Copper =o number of protons of electrons Symbols used=tonumber represent the elements. Examples: o Nitrogen – N o The symbols have 1, 2 or 3 letters o Calcium – Ca o The first letter is ALWAYS CAPATALIZED o Magnesium – Mg o Any other letters are ALWAYS LOWER CASE o Carbon - C Chemical Formulas o Chemical symbols put together to represent a compound. o The chemical formula for water is H2O. o The 2 is a subscript. The subscript tells us how many atoms there are of the element IN FRONT OF IT. Chemical Name 64 + number of neutrons Chemical Symbols Chemical Symbol Examples: o Water = H2O: 2 Hydrogens, 1 Oxygen o Salt = NaCl: 1 Sodium, 1 Chlorine o Glucose = C6H12O6: 6 Carbons, 12 Hydrogens, 6 Oxygens Name the elements that are in the following compounds: H2SO4 There are 2______________________ atoms, 1 ______________________ atom, and 4 ______________________ atoms NaHCO3 There is 1_____________ atom, 1______________ atom, 1 ______________ atom, and 3 ______________ atoms How many atoms of each element are present in the following compounds? HC2H3O2 There are ___ atoms of hydrogen, ___ atoms of carbon, and ___ atoms of oxygen CaCO3 There are ___ atom of calcium, ___ atom of carbon, and ___ atoms of oxygen