PHAR2811 Lecture Amino acids as drug targets
advertisement

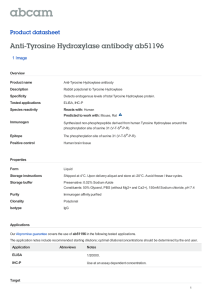
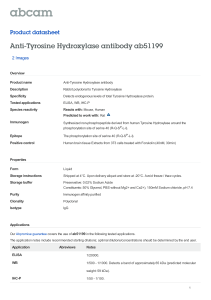
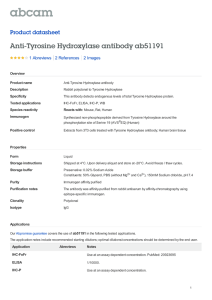
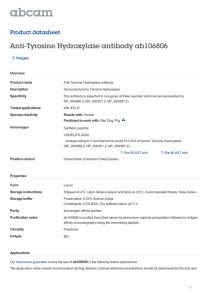
PHAR2811 Lecture Amino acids as drug targets COMMONWEALTH OF AUSTRALIA Copyright Regulation WARNING This material has been reproduced and communicated to you by or on behalf of the University of Sydney pursuant to Part VB of the Copyright Act 1968 (the Act). The material in this communication may be subject to copyright under the Act. Any further reproduction or communication of this material by you may be the subject of copyright protection under the Act. Do not remove this notice Amino Acid derivatives • • • • • • Adrenalin/epinephrine Serotonin GABA Histamine Dopa & dopamine thyroxin The Catecholamine Family • L-dopa • Dopamine • Noradrenalin or Norepinephrine • Adrenalin or epinephrine OH OH Catechol The Catecholamine Family O O H3N+ CH C H3N+ O- CH 2 CH 2 Tyrosine hydroxylase OH Tyrosine CH HO OH L-Dopa C O- The Catecholamine Family CH 3 +NH3 CH 2 decarboxylation CH 2 hydroxylation HO +NH3 +NH2 CH 2 CH 2 CH HO HO OH L-Dopamine HO methylation CH HO OH Noradrenalin OH Adrenalin The Catecholamine Family • Family members act as neurotransmitters in the brain and hormones in the circulatory system • Produced in adrenal medulla and sympathetic neurons • The pools are kept separate by the blood brain barrier Acting as hormones • The flight or fight response • Adrenalin and noradrenalin are produced in the adrenal medulla and stored in granules • Released into the circulation by stimuli from the sympathetic nervous system • They bind to specific receptors Adrenergic receptors • Glycoproteins which span the membrane • Known as G-coupled protein receptors • 4 classes of adrenergic receptors: a1, a2, b1, b2 • Some are stimulatory (β) some inhibitory (α) • Activate or inhibit adenylyl cyclase cAMP Adrenergic receptors • Have short term and long term effects • The alpha receptors stimulate smooth muscle contraction in peripheral organs • the beta receptors mobilise fuels, relax smooth muscles of the bronchi and blood vessels supplying skeletal muscles and increase heart rate. Adrenergic receptors • The end result of these actions is to mobilise and shunt energy reserves to where they are most needed • prepare for action! Used as a drug: Adrenalin • Treatment for cardiac arrest and anaphylactic reactions • Bronchodilator properties used in asthma • Agonists and antagonists to adrenergic receptors are also used as drugs Acting as neurotransmitters • Noradrenalin is uniquely found between the junctions of sympathetic neurons and smooth muscle cells • Decreased levels of noradrenalin in the brain are associated with some forms of clinical depression Noradrenalin and depression Strategies to increase noradrenalin levels in the brain: • Inhibit inactivation (monoamine oxidase inhibitors or MAO inhibitors) OR • Inhibit reuptake (tricyclics) Other Catecholamines • L-dopa and dopamine • Dopamine is also a neurotransmitter in synapses in localised areas of the brain stem • Parkinson’s disease is caused from the degeneration of these dopaminergic neurons. • The psychotic symptoms of Schizophrenia are associated with elevated dopamine Parkinson’s disease • Parkinson patients are treated with L-dopa or levadopa • Although it is dopamine that is deficient it cannot cross the blood brain barrier • L-dopa crosses the barrier (on amino acid transporters), where it is decarboxylated to produce dopamine • It is usually administered with a peripheral decarboxylase inhibitor; carbidopa (to prevent the LDopa going to dopamine before it gets to the brain). L-Dopa and dopamine +NH3 O H3N+ CH CH 2 Crosses the blood brain barrier HO OH L-Dopa C O- CH 2 CH 2 decarboxylation This reaction must be inhibited before it gets to the blood brain barrier HO OH L-Dopamine carbidopa HNH 2N CH 3 O C C O OH H3N+ CH 2 HO CH CH 2 HO OH Carbidopa, an inhibitor of levadopa decarboxylation OH L-Dopa C O- Tyrosine Hydroxylase (TH) • The rate limiting step in the pathway • Adding another –OH to the aromatic ring • It requires O2 and biopterin (this moiety also makes up folates – we obtain it from our diet or microorganisms in the gut) • This is a tricky reaction • There are only a few examples of this in life! Tyrosine Hydroxylase O O H3N+ CH CH 2 C H3N+ O- OH Tyrosine CH 2 O2 Tyrosine hydroxylase CH HO OH L-Dopa C O- Biopterin H2N H N N OH N OH O CH 3 N H OH Tyrosine hydroxylase • Although it is the rate limiting step in synthesis it is not a great site for drug action. • There are drugs that inhibit the enzyme but these are rarely used • Regulation of adrenalin is done at the release phase • Most drugs work on the receptor Tyrosine hydroxylase • There are 3 isoforms produced by alternative splicing although the biological significance of these is not entirely clear. • Another example of a similar reaction is the conversion of phenylalanine to tyrosine • The enzyme here is phenylalanine hydroxylase • This reaction also requires biopterin Phenylketonuria (PKU) • The conversion of phenylalanine to tyrosine is a similar hydroxylation • Catalysed by phenylalanine hydroxylase • If this enzyme is defective the phenylalanine has to go elsewhere • Accumulates as phenylpyruvate Phenylalanine hydroxylase O H3N+ CH C O O- O2 CH 2 H3N+ CH CH 2 Phenylalanine hydroxylase Phenylalanine OH Tyrosine C O- PKU: diversion O H3N+ CH C CH 2 O O- Oxidative deamination O C C CH 2 Build up causes severe mental retardation in developing brain Phenylalanine Phenylpyruvate O- PKU diet Thyroxin I I +NH3 HO H2 C O CH C I I O- Synthesised from 2 tyrosine residues O Thyroxin Protein, thyroglobulin CH 2 N OH HO C H2 C Thyroxin CH 2 I I OH I Iodination Oxidative coupling H2 C HO I I I +NH3 Thyroxin HO H2 C O CH C I I I I O O- +NH3 HO H2 C O CH C I O I O- I I +NH3 HO I O O CH C I I proteolysis HO H2 C O I O- CH 2 I I +NH3 I I HO H2 C O CH C I O I O- I I +NH3 HO H2 C O CH C I I O- 5 – 6 thyroxins released per thyroglobulin O Hypothyroidism: • thyroxin levels too low; individuals present with lethargy, cold skin and often overweight. • low iodine in the diet often the result of low iodine in the soil….leads to a goiter Cretinism • Thyroxin is essential for normal growth and development • A deficiency will result in cretinism, a condition defined by mental retardation, stunted growth Serotonin • A derivative synthesised by the decarboxylation of tryptophan. • A neurotransmitter • low levels of serotonin have also been linked to depression. • Prozac acts to inhibit the degradation of serotonin. Synthesis of Serotonin O H3N+ CH C O- H3N+ CH 2 CH 2 CH 2 OH HN HN Tryptophan Serotonin Histamine • Produced by the decarboxylation of histidine • A common product of allergic reactions, produced by mast cells • Neurotransmitter • Involved in sleep regulation • Regulates acid secretions in the stomach • Antihistamines are used to relieve the symptoms of an allergic reaction Histamine synthesis O H3N+ CH C O- H3N+ CH 2 CH 2 N decarboxylation NH Histidine CH 2 N NH Histamine GABA • Gamma amino butyric acid • Produced by the decarboxylation of glutamate • Major inhibitory neurotransmitter • Released by 30% of synapses GABA synthesis O H3N+ CH C +NH3 O- CH 2 CH 2 CH 2 CH 2 CH 2 C OH Glutamate O Decarboxylation C OH GABA O Amino Acid derivatives • • • • • • Adrenalin/epinephrine Serotonin GABA Histamine Dopa & dopamine thyroxin