Botany: Part III Plant Nutrition

Botany: Part III
Plant Nutrition
H
2
O
Figure 36.2-1
H
2
O and minerals
Plant Nutrition and
Transport
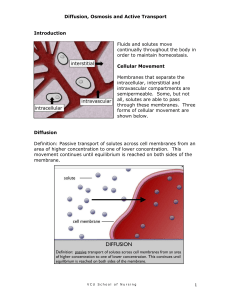
Water and minerals in the soil are absorbed by the roots.
Transpiration, the loss of water from leaves
(mostly through stomata), creates a force within leaves that pulls xylem sap upward.
Transpiration
3
Getting Water Into The Xylem Of The Root
4
Generation of Transpirational Pull
In addition to apoplastic and symplastic movement, there are newly discovered channels called
aquaporins that allow
only water to move across the membrane.
Water movement through aquaporins is quicker since no lipids are involved.
5
Movement of Minerals
Into The Root
Plants need minerals to synthesize organic compounds such as amino acids, proteins and lipids.
Plants obtain these minerals from the soil and are transported by various transport proteins.
6
Macro- and Micro- Nutrients
Macronutrients are required by plants in relatively large amounts and compose much of the plant’s structure.
(C, N, O, P, S, H, K, Ca,
Mg, Si, etc. )
Micronutrients are needed in very small quantities. Typically function as cofactors.
7
Mycorrhizae: A Mutualistic Relationship
Roots
Fungus
H
2
O
H
2
O and minerals
2
O
2
• Gas exchange occurs through the stomata.
• CO
2 is required for photosynthesis and O
2 is released into the atmosphere.
O
2
CO
2
• Roots exchange gases with the air spaces in the soil, taking in O
2 and releasing CO
2
.
H
2
O
H
2
O and minerals
2
O
2
Sugar
Light
• Sugars are produced by photosynthesis in the leaves.
O
2
CO
2
• Phloem sap(green arrows) can flow both ways.
• Xylem sap(blue arrows) transport water and minerals upward from roots to shoots.
Water Is In The Root, So Now What?
• Root pressure is caused by active distribution of mineral nutrient ions into the root xylem.
• Without transpiration to carry the ions up the stem, they accumulate in the root xylem and lower the water potential.
• At night in some plants, root pressure causes guttation or exudation of drops of xylem sap from the tips or edges of leaves as pictured here.
11
Water Is In The Root, So Now What?
• Water then diffuses from the soil into the root xylem due to osmosis.
• Root pressure is caused by this accumulation of water in the xylem pushing on the rigid cells.
• Root pressure provides a force, which pushes water up the stem, but it is not enough to account for the movement of water to leaves at the top of the tallest trees.
12
Let’s Apply Some TACT To The Situation!
A more likely scenario involves the Cohesion-Tension Theory
( also known as Tension-Adhesion-Cohesion-Transpiration or TACT Theory)
T ension
: Water is a polar molecule.
When two water molecules approach one another they form an intermolecular attraction called a hydrogen bond.
This attractive force, along with other intermolecular forces, is one of the principal factors responsible for the occurrence of surface tension in liquid water.
It also allows plants to draw water from the root through the xylem to the leaf.
13
Let’s Apply Some TACT To The Situation!
• Adhesion occurs when water forms hydrogen bonds with xylem cell walls.
• Cohesion occurs when water molecules hydrogen bond with each other.
14
Let’s Apply Some TACT To The Situation!
• Transpiration: Water is constantly lost by transpiration in the leaf.
• When one water molecule is lost another is pulled along by the processes of cohesion and adhesion.
• Transpiration pull, utilizing capillary action and the inherent surface tension of water, is the primary mechanism of water movement in plants.
15
Generation of Transpiration Pull
16
Ode To The Hydrogen Bond
Water Potential
• Water potential quantifies the tendency of free (not bound to solutes) water to move from one area to another due to osmosis, gravity, mechanical pressure, or matrix effects such as surface tension.
• Water potential has proved especially useful in understanding water movement within plants, animals, and soil.
• Water potential is typically expressed in potential
energy per unit volume and very often is represented by the Greek letter psi,
.
(pronounced as “sigh” )
Water Potential
• The addition of solutes to water lowers the
water's potential (makes it more negative), just as the increase in pressure increases its potential (makes it more positive).
• Pure water is usually defined as having an osmotic potential (
) of zero, and in this case, solute potential can never be positive.
• Free water moves from regions of higher water potential to regions of lower water
potential if there is no barrier to its flow.
Water Potential
• The word “potential” refers to water’s potential
energy which is water’s capacity to perform work when it moves from a region of higher water
potential to a region of lower water potential.
• The water potential equation is
=
S where
is the water potential,
S
+
P is the solute potential (directly proportional to its molarity and sometimes called the osmotic potential and the
S of pure water is zero) and
P is the pressure potential.
Water Potential
•
P is the physical pressure exerted on a solution.
• It can be either positive or negative relative to the atmospheric pressure.
• Water in a nonliving hollow xylem cells is under a negative potential (tension) of less than
−2 MPa.
• BUT the water in a living cell is usually under positive pressure due to the osmotic uptake of water.
Solutes have a negative effect on
by binding water molecules.
Pure water at equilibrium
Positive pressure has a positive effect on
by pushing water.
Pure water at equilibrium
Solutes and positive pressure have opposing effects on water movement.
Pure water at equilibrium
Negative pressure
(tension) has a negative effect on
by pulling water.
Pure water at equilibrium
H
2
O
Adding solutes to the right arm makes
lower there, resulting in net movement of water to the right arm:
H
2
O H
2
O H
2
O
Applying positive pressure to the right arm makes
higher there, resulting in net movement of water to the left arm:
Positive pressure
In this example, the effect of adding solutes is offset by positive pressure, resulting in no net movement of water:
Positive pressure
Applying negative pressure to the right arm makes
lower there, resulting in net movement of water to the right arm:
Negative pressure
Pure water
Membrane
H
2
O
Solutes
H
2
O H
2
O
Solutes
H
2
O
Solutes have a negative effect on
by binding water molecules.
Pure water at equilibrium
Positive pressure has a positive effect on
by pushing water.
Pure water at equilibrium
Solutes and positive pressure have opposing effects on water movement.
Pure water at equilibrium
Negative pressure
(tension) has a negative effect on
by pulling water.
Pure water at equilibrium
H
2
O
Adding solutes to the right arm makes
lower there, resulting in net movement of water to the right arm:
H
2
O H
2
O H
2
O
Applying positive pressure to the right arm makes
higher there, resulting in net movement of water to the left arm:
Positive pressure
In this example, the effect of adding solutes is offset by positive pressure, resulting in no net movement of water:
Positive pressure
Applying negative pressure to the right arm makes
lower there, resulting in net movement of water to the right arm:
Negative pressure
Pure water
Membrane
H
2
O
Solutes
H
2
O H
2
O
Solutes
H
2
O
Solutes have a negative effect on
by binding water molecules.
Pure water at equilibrium
Positive pressure has a positive effect on
by pushing water.
Pure water at equilibrium
Solutes and positive pressure have opposing effects on water movement.
Pure water at equilibrium
Negative pressure
(tension) has a negative effect on
by pulling water.
Pure water at equilibrium
H
2
O
Adding solutes to the right arm makes
lower there, resulting in net movement of water to the right arm:
H
2
O H
2
O H
2
O
Applying positive pressure to the right arm makes
higher there, resulting in net movement of water to the left arm:
Positive pressure
In this example, the effect of adding solutes is offset by positive pressure, resulting in no net movement of water:
Positive pressure
Applying negative pressure to the right arm makes
lower there, resulting in net movement of water to the right arm:
Negative pressure
Pure water
Membrane
H
2
O
Solutes
H
2
O H
2
O
Solutes
H
2
O
Solutes have a negative effect on
by binding water molecules.
Pure water at equilibrium
Positive pressure has a positive effect on
by pushing water.
Pure water at equilibrium
Solutes and positive pressure have opposing effects on water movement.
Pure water at equilibrium
Negative pressure
(tension) has a negative effect on
by pulling water.
Pure water at equilibrium
H
2
O
Adding solutes to the right arm makes
lower there, resulting in net movement of water to the right arm:
H
2
O H
2
O H
2
O
Applying positive pressure to the right arm makes
higher there, resulting in net movement of water to the left arm:
Positive pressure
In this example, the effect of adding solutes is offset by positive pressure, resulting in no net movement of water:
Positive pressure
Applying negative pressure to the right arm makes
lower there, resulting in net movement of water to the right arm:
Negative pressure
Pure water
Membrane
H
2
O
Solutes
H
2
O H
2
O
Solutes
H
2
O
Water Potential vs. Tonicity
26
Water Potential and Plant Vocabulary
The green arrows indicate water moving OUT of the cell.
The arrows indicate water moving INTO the cell.
27
Once More With Feeling!
Initial conditions: cellular
greater than environmental
0.4 M sucrose solution:
P
S
= 0
= − 0.9
=
−
0.9 MPa
Initial flaccid cell:
= 0
P
S
= −0.7
=
−
0.7 MPa
Plasmolyzed cell at osmotic equilibrium with its surroundings
P
S
= 0
= − 0.9
=
−
0.9 MPa
Last Time, I Promise!
Initial conditions: cellular
less than environmental
Initial flaccid cell:
P
= 0
S
= − 0.7
=
−
0.7 MPa
Distilled water:
P
= 0
S
= 0
= 0 MPa
Turgid cell at osmotic equilibrium with its surroundings
P
S
= 0.7
= − 0.7
=
−
0 MPa
Wilting
• Turgor loss in plants causes wilting
– Which can be reversed when the plant is watered
Ascent of Xylem Sap
31
Stomata Regulate Transpiration Rate
• When water moves into guard cells from neighboring cells by osmosis, they become more turgid.
• The structure of the guard cells’ wall causes them to bow outward in response to the incoming water.
• This bowing increases the size of the pore (stomata) between the guard cells allowing for an increase in gas exchange.
32
Homeostasis and Water Regulation
• By contrast, when the guard cells lose water and become flaccid, they become less bowed , and the pore
(stomata) closes.
• This limits gas exchange.
33
Role Of Potassium Ion In
Stomatal Opening And Closing
The transport of K + (potassium ions, symbolized here as red dots) across the plasma membrane and vacuolar membrane causes the turgor changes of guard cells.
H
2
O
H
2
O
H
2
O H
2
O
H
2
O
K +
H
2
O
H
2
O
H
2
O H
2
O H
2
O
34
Homeostasis and Water Balance
• Trees that experience a prolonged drought may compensate by losing part of their crown as a consequence of leaves dying and being shed.
• Resources may be reallocated so that more energy is expended for root growth in the “search” for additional water.
35
Natural Selection and Arid Environments
36
Natural Selection and Arid Environments
Plants that have adapted to arid environments have the following leaf adaptations:
1. Leaves that are thick and hard with few stomata placed only on the underside of the leaf
2. Leaves covered with trichomes (hairs) which reflect more light thus reducing the rate of transpiration
3. Leaves with stomata located in surface pits which increases water tension and reduces the rate of transpiration photosynthesis (cacti) and store water.
Natural Selection and Flooding
• Plants that experience prolonged flooding will have problems.
• Roots underwater cannot obtain the oxygen needed for cell respiration and ATP synthesis.
• As a result, leaves may dry out causing the plant to die.
• Additionally, production of hormones that promote root synthesis are suppressed.
38
Adaptations to Water Environments
39
Adaptations to Water Environments
Plants that have adapted to wet environments have the following adaptations:
1. Formation of large lenticels (pores) on the stem.
2. Formation of adventitious roots above the water that increase gas exchange.
3. Formation of stomata only on the surface of the leaf (water lilies).
4. Formation of a layer of air-filled channels called
aerenchyma for gas exchange which moves gases between the plant above the water and the submerged tissues.
40
Bulk Flow of Photosynthetic Products
Vessel
( xylem )
H
2
O
Sieve tube
(phloem)
1
2
Source cell
(leaf)
Sucrose
H
2
O
4
H
2
O
Sink cell
(storage root)
3
Sucrose
1 Loading of sugar (green dots) into the sieve tube at the source reduces water potential inside the sieve-tube members. This causes the tube to take up water by osmosis.
2
This uptake of water generates a positive pressure that forces the sap to flow along the tube.
4
3
The pressure is relieved by the unloading of sugar and the consequent loss of water from the tube at the sink.
In the case of leaf-to-root translocation, xylem recycles water from sink to source.
41
Nutritional Adaptations in Plants
• Epiphytes- grow on other plants, but do not harm their host
• Parasitic Plants-absorb water, minerals, and sugars from their host
• Carnivorous Plantsphotosynthetic but supplement their mineral diet with insects and small animals; found in nitrogen poor soils
42
Halophytes
43
Adaptations of Plants: Saline Environments
• Soil salinity around the world is increasing.
• Many plants are killed by too much salt in the soil.
• Some plants are adapted to growing in saline conditions (halophytes)
• Have spongy leaves with water stored that dilutes salt in the roots
• Actively transport the salt out of the roots or block the salt so that it cannot enter the roots
• Produce high concentrations of organic molecules in the roots to alter the water potential gradient of the roots
44
Created by:
Jackie Snow
AP Biology Teacher and Instructional Facilitator, Belton ISD
Belton, TX