Reaction types
advertisement

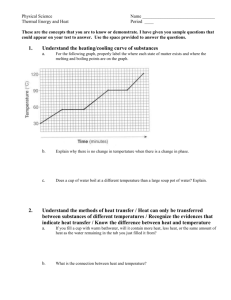
Reaction types Types of reactions 7.2 Types of Reactions • 7 types of reactions • Synthesis: is a reaction in which two or more substances react to form a more complex single substance • A+BC • Ex. 2 Na + Cl2 2 NaCl • Decomposition Reaction: is a reaction in which a compound breaks down into two or more simpler substances • ABA+B • Ex. 2 H20 2 H2 + O2 • Compost pile, digesting food, electrolysis (breaking down H2O with electricity) • Combustion Reaction: is reaction where a substance reacts rapidly with oxygen. • Flammability or explosiveness • Ex. CH4 + 2 O2 CO2 + 2 H2O • Ex. Burning any type of fuel • Usually produces CO2 (greenhouse gas) • Oxidation Reaction: are reactions where a substance reacts slowly with the oxygen in air or water • Happens with metals • “rust” or “tarnish” • Ex. 2 Ca + O2 2 CaO • Single Replacement Reaction: is a reaction where one element takes the place of another element • A+BC AC+B or D+BC BD +C • Cu + 2 AgNO3 2 Ag + Cu(NO3)2 • Double Replacement: is a reaction where 2 elements replace each other • AB+CDAD+CB • CaCO3 + 2 HCl CaCl2 + H2CO3 Discussion Question: • What two Chemical reactions are “opposites” of each other and why? • Synthesis and decomposition; in synthesis multiple substances combine to form a new one, while decomposition a single substance breaks apart into multiple simpler substances • Exothermic Reactions: are reactions that release energy into their surroundings • Give off heat (exergonic) • Ex. Combustion Reactions – Ex. Burning fossil fuels • Endothermic Reaction: is a reaction where heat energy is absorbed by its surroundings • Ice absorbs heat to melt into water • Gets colder (endergonic) • Ex. Ice pack and decomposition of mercury Energy and reaction rates http://images.slideplayer.com/15/4724806/slides/slide_2.jpg Reaction Rates • • • • • • Increasing Reaction Rate: 1. Temperature 2. Surface area 3. Stirring 4. Concentration of Reactants 5. Catalyst: is a substance that affects reaction rate without being used up. • Activation energy: What it takes to get a reaction started • Inhibitor: Something that stops or inhibits a reaction from proceeding • Rate of reaction: how fast a reaction proceeds Discussion Question: • How are chemical bonds involved in energy exchanges? • Breaking bonds requires energy; forming bonds releases energy. What affects the rate of a reaction • Heat • Size of particle/ surface area • Catalysts What is a catalyst? • A substance that speeds up a reaction without being changed by the reaction. • Enzymes are biological or protein catalysts. Reaction Energy All chemical reactions are accompanied by a change in energy. Exothermic - reactions that release energy to their surroundings (usually in the form of heat) oΔH (enthalpy) is negative – energy leaving system Endothermic - reactions that need to absorb heat from their surroundings to proceed. oΔH (enthalpy) is positive – energy coming into the system Reaction Energy •Spontaneous Reactions - Reactions that proceed immediately when two substances are mixed together. Not all reactions proceed spontaneously. •Activation Energy – the amount of energy that is required to start a chemical reaction. •Once activation energy is reached the reaction continues until you run out of material to react. http://www.docbrown.info/page03/3_31rates/Image35.gif https://dr282zn36sxxg.cloudfront.net/datastreams/fd%3Ad07bc599a7310d9fd6fbfea4b44861cb93fdcd869142ecfb61bc2b4b%2BIMAGE_ THUMB_POSTCARD%2BIMAGE_THUMB_POSTCARD.1 http://pipeliningsupply.com/wp-content/uploads/2015/09/Exotherm.gif https://dr282zn36sxxg.cloudfront.net/datastreams/fd%3Ad07bc599a7310d9fd6fbfea4b44861cb93fdcd869142ecfb61bc2b4b%2BIMAGE_ THUMB_POSTCARD%2BIMAGE_THUMB_POSTCARD.