Acid-Base Equilibria Chapter 16
advertisement

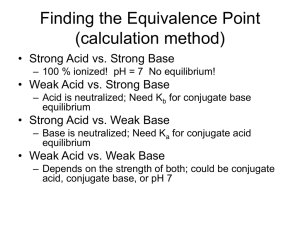
Acid-Base Equilibria Chapter 16 The common ion effect is the shift in equilibrium caused by the addition of a compound having an ion in common with the dissolved substance. The presence of a common ion suppresses the ionization of a weak acid or a weak base. Consider mixture of CH3COONa (strong electrolyte) and CH3COOH (weak acid). CH3COONa (s) Na+ (aq) + CH3COO- (aq) CH3COOH (aq) H+ (aq) + CH3COO- (aq) common ion 16.2 What is the pH of a solution containing 0.30 M HCOOH and 0.52 M HCOOK? Mixture of weak acid and conjugate base! HCOOH (aq) Initial (M) Change (M) Equilibrium (M) 0.30 0.00 0.52 -x +x +x 0.30 - x x 0.52 + x Ka for HCOOH = 1.8 x 10 -4 [H+] [HCOO-] Ka = H+ (aq) + HCOO- (aq) x = 1.038 X 10 -4 pH = 3.98 [HCOOH] 16.2 OR…… Use the Henderson-Hasselbach equation Consider mixture of salt NaA and weak acid HA. NaA (s) Na+ (aq) + A- (aq) HA (aq) H+ (aq) + A- (aq) [H+] Ka [HA] = [A-] -log [H+] = -log Ka - log [HA] [A-] -] [A -log [H+] = -log Ka + log [HA] [H+][A-] pK = -log K Ka = a a [HA] Henderson-Hasselbach equation [conjugate base] pH = pKa + log [acid] [A-] pH = pKa + log [HA] 16.2 What is the pH of a solution containing 0.30 M HCOOH and 0.52 M HCOOK? Mixture of weak acid and conjugate base! HCOOH (aq) Initial (M) Change (M) Equilibrium (M) Common ion effect 0.30 – x 0.30 0.52 + x 0.52 H+ (aq) + HCOO- (aq) 0.30 0.00 0.52 -x +x +x 0.30 - x x 0.52 + x [HCOO-] pH = pKa + log [HCOOH] [0.52] = 4.01 pH = 3.77 + log [0.30] HCOOH pKa = 3.77 16.2 A buffer solution is a solution of: 1. A weak acid or a weak base and 2. The salt of the weak acid or weak base Both must be present! A buffer solution has the ability to resist changes in pH upon the addition of small amounts of either acid or base. Consider an equal molar mixture of CH3COOH and CH3COONa CH3COOH (aq) H+ (aq) + CH3COO- (aq) Adding more acid creates a shift left IF enough acetate ions are present 16.3 HCl HCl + CH3COO- H+ + ClCH3COOH + Cl- 16.3 Chemistry In Action: Maintaining the pH of Blood 16.3 Titrations In a titration a solution of accurately known concentration is added gradually added to another solution of unknown concentration until the chemical reaction between the two solutions is complete. Equivalence point – the point at which the reaction is complete Indicator – substance that changes color at the endpoint (hopefully close to the equivalence point) Slowly add base to unknown acid UNTIL The indicator changes color (pink) 4.7 Strong Acid-Strong Base Titrations NaOH (aq) + HCl (aq) OH- (aq) + H+ (aq) H2O (l) + NaCl (aq) H2O (l) 100% ionization! No equilibrium 16.4 Weak Acid-Strong Base Titrations CH3COOH (aq) + NaOH (aq) CH3COONa (aq) + H2O (l) CH3COOH (aq) + OH- (aq) CH3COO- (aq) + H2O (l) At equivalence point (pH > 7): CH3COO- (aq) + H2O (l) OH- (aq) + CH3COOH (aq) 16.4 Strong Acid-Weak Base Titrations HCl (aq) + NH3 (aq) H+ (aq) + NH3 (aq) NH4Cl (aq) NH4Cl (aq) At equivalence point (pH < 7): NH4+ (aq) + H2O (l) NH3 (aq) + H+ (aq) 16.4 Acid-Base Indicators 16.5 pH 16.5 The titration curve of a strong acid with a strong base. 16.5 Which indicator(s) would you use for a titration of HNO2 with KOH ? Weak acid titrated with strong base. At equivalence point, will have conjugate base of weak acid. At equivalence point, pH > 7 Use cresol red or phenolphthalein 16.5 Finding the Equivalence Point (calculation method) • Strong Acid vs. Strong Base – 100 % ionized! pH = 7 No equilibrium! • Weak Acid vs. Strong Base – Acid is neutralized; Need Kb for conjugate base equilibrium • Strong Acid vs. Weak Base – Base is neutralized; Need Ka for conjugate acid equilibrium • Weak Acid vs. Weak Base – Depends on the strength of both; could be conjugate acid, conjugate base, or pH 7 Exactly 100 mL of 0.10 M HNO2 are titrated with 100 mL of a 0.10 M NaOH solution. What is the pH at the equivalence point ? start (moles) 0.01 0.01 HNO2 (aq) + OH- (aq) NO2- (aq) + H2O (l) end (moles) 0.0 0.0 0.01 Final volume = 200 mL NO2- (aq) + H2O (l) Initial (M) Change (M) 0.01 = 0.05 M 0.200 OH- (aq) + HNO2 (aq) [NO2-] = 0.05 0.00 0.00 -x +x +x x x Equilibrium (M) 0.05 - x [OH-][HNO2] x2 -11 = 2.2 x 10 Kb = = [NO2-] 0.05-x pOH = 5.98 0.05 – x 0.05 x 1.05 x 10-6 = [OH-] pH = 14 – pOH = 8.02 Complex Ion Equilibria and Solubility A complex ion is an ion containing a central metal cation bonded to one or more molecules or ions. Co2+ (aq) + 4Cl- (aq) Co(H2O)2+ 6 CoCl42- (aq) CoCl24 16.10 16.10 Complex Ion Formation • These are usually formed from a transition metal surrounded by ligands (polar molecules or negative ions). • As a "rule of thumb" you place twice the number of ligands around an ion as the charge on the ion... example: the dark blue Cu(NH3)42+ (ammonia is used as a test for Cu2+ ions), and Ag(NH3)2+. • Memorize the common ligands. Common Ligands Ligands Names used in the ion H2O NH3 aqua ammine OHClBrCNSCN- hydroxy chloro bromo cyano thiocyanato (bonded through sulphur) isothiocyanato (bonded through nitrogen) Names • Names: ligand first, then cation Examples: – tetraamminecopper(II) ion: Cu(NH3)42+ – diamminesilver(I) ion: Ag(NH3)2+. – tetrahydroxyzinc(II) ion: Zn(OH)4 2- • The charge is the sum of the parts (2+) + 4(-1)= -2. Coordination Number • Total number of bonds from the ligands to the metal atom. • Coordination numbers generally range between 2 and 12, with 4 (tetracoordinate) and 6 (hexacoordinate) being the most common. Some Coordination Complexes molecular formula Lewis base/ligand Lewis acid donor atom coordination number Ag(NH3)2+ NH3 Ag+ N 2 [Zn(CN)4]2- CN- Zn2+ C 4 [Ni(CN)4]2- CN- Ni2+ C 4 [PtCl6] 2- Cl- Pt4+ Cl 6 Ni2+ N 6 [Ni(NH3)6]2+ NH3 When Complexes Form • Aluminum also forms complex ions as do some post transitions metals. Ex: Al(H2O)63+ • Transitional metals, such as Iron, Zinc and Chromium, can form complex ions. • The odd complex ion, FeSCN2+, shows up once in a while • Acid-base reactions may change NH3 into NH4+ (or vice versa) which will alter its ability to act as a ligand. • Visually, a precipitate may go back into solution as a complex ion is formed. For example, Cu2+ + a little NH4OH will form the light blue precipitate, Cu(OH)2. With excess ammonia, the complex, Cu(NH3)42+, forms. • Keywords such as "excess" and "concentrated" of any solution may indicate complex ions. AgNO3 + HCl forms the white precipitate, AgCl. With excess, concentrated HCl, the complex ion, AgCl2-, forms and the solution clears.