Human Subjects and IRB Review General Guidelines and Data Handling
advertisement

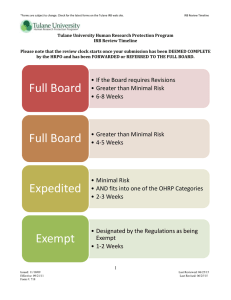
Human Subjects and IRB Review General Guidelines and Data Handling The approach to protecting human subjects is guided by the ethical principles and guidelines outlined in the Belmont Report: respect for persons, beneficence, and justice. These ethical principles are legally codified in the human subjects protection and regulations for the Department of Health and Human Services (DHHS), under Title 45 CFR Part 46. Subpart A of these regulations has been accepted as the “Common Rule” by many other federal agencies that sponsor human subjects research. Tulane University holds Federal-wide Assurances [Tulane University FWA No. 00002055) with the DHHS Office for Human Research Protections (OHRP). These assurances are our formal agreements with OHRP to ensure that all of our human subjects research is conducted in accordance with 45 CFR 46 (and the FDA regulations, where applicable), including the requirement for Institutional Review Board (IRB) review and approval. In addition to the federal regulations, we take into consideration any state or local laws regarding human subjects that may be more protective than the federal statutes. Tulane University maintains an IRB that is responsible for reviewing and approving any human subject research in which their staff are engaged, following the applicable regulations. All IRB members are experienced and up-to-date in IRB issues. The Board contains Certified IRB Professionals (CIP designations). The IRB meets regularly to provide timely review of all new and ongoing projects.