Bohr’s model of H atom PHY123 7/24/2016 Lecture XIII
advertisement

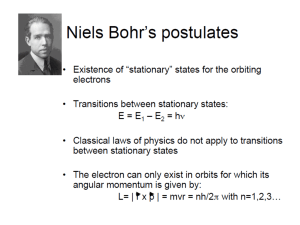
Bohr’s model of H atom PHY123 7/24/2016 Lecture XIII 1 Niels Bohr (1885-1962) 7/24/2016 Lecture XIII 2 By Iutta Waloschek Hydrogen atom • Positively charged nucleus inside (+Ze), negatively charged electron (-e) around • Electron is attracted to nucleus • Electron is trapped in a potential well created by nucleus (“a box”) Ze 2 U eV 40 r 1 • Energy levels in atom 7/24/2016 Lecture XIII 3 Standing electron waves in Hydrogen atom • Standing waves: • 2rn=nl lh/mv mvrn=nh/2 • Angular momentum L=mvrn is quantized L=nh/2 • n – orbital quantum number 7/24/2016 Lecture XIII 4 Disclaimer • Though Bohr’s model was able to predict many properties of H atom and correctly calculate some of its characteristics, this model is incomplete and it is not advisable to think of an atom as a miniature “solar system”. • Orbits make sense only as average radii of electron position (not the same as electron slightly smeared around circular orbits!!!) 7/24/2016 Lecture XIII 5 Hydrogen atom • mvrn=nh/2 v=nh/(2mrn) • Newton’s 2nd law for circular motion: mv 2 F r 1 Ze 2 mv 2 n2h2 2 40 r r 4 2 mr 3 n 2 h 2 0 n 2 rn r1 2 mZe Z h 2 0 10 r1 0 . 529 10 m 2 me 7/24/2016 Bohr radius Lecture XIII 6 Hydrogen atom • Energy in H atom KE+U mv m nh m Z e KE 2 2 4 2 m 2 n 4 h 4 02 2 2 Z 2e 4 m KE 2 2 2 8n h 0 Ze 2 U eV 40 r 1 Z 2e 4 m U 2 2 2 2 KE 4n h 0 7/24/2016 2 2 2 2 4 Z 2e 4 m En KE U KE 2 2 2 8n h 0 Z2 En 2 E1 n e4m E1 2 2 13.6eV 8h 0 Lecture XIII 7 Hydrogen atom • What did we learn? • For high n energy approaches -0, radius approaches infinity • Energy proportional to Z2e4 – Ze2 from the potential, Ze2 from 1/r (smaller orbits around larger charges) • Radius inversely proportional to mass (F=mv2/r) Energy proportional to electron mass • Energy does not depend on nucleus mass – assume infinitely heavy • In classical case (h0) rn0 – electron falls on nucleus. 7/24/2016 Lecture XIII n 2 h 2 0 rn mZe2 Z 2e 4 m En 2 2 2 8n h 0 8 Hydrogen atom • Energy levels in H 13.6 En 2 eV n • Infinite number of states n=1infinity • All energies are negative – electron is trapped in atom • Lowest possible energy level E1=-13.6 eV – ground state • Positive energy – free electron –ionization • Ionization energy =13.6/n2 for atom in n state 7/24/2016 Lecture XIII 9 Some parameters of the H atom • Ground state n=1, Z=1 • Electron’s “orbit” radius h 2 0 10 r1 0 . 529 10 m 2 me • Wavelength l 2r 3.11010 m 0.31nm 1 • Momentum p h / l hc / lc 1240eV nm / 0.31nm / c 4 KeV / c • v/c p mc 2 v / c 2 4 KeV / c 0.5 10 6 eV v / c 4 103 eV v / c 8 10 3 7/24/2016 Lecture XIII 10 Some parameters of the H atom • Ground state n=1, Z=1 4 e m • Kinetic Energy KE 13.6eV 2 2 8h 0 • Potential Energy e4m U 2 2 27.2eV 4h 0 e4m • Total energy E 2 2 13.6eV 8h 0 • Longest wavelength absorbed (l – max E =hc/l – min) n=1n=2 E1=-13.6eV E2=-13.6/4=-3.4eV Eg=13.6-3.4=10.2eV lhc/Eg1243eVnm/10.2eV=122nm 7/24/2016 Lecture XIII 11 A cute problem • Electrons are accelerated by 12.3V potential difference and pass through hydrogen gas at room T (ground state n=1). Which wavelengths of light can be observed? 7/24/2016 Lecture XIII 12