HUMAN SUBJECT RESEARCH COMPLIANCE July 23, 2013 PRESENTED BY BRIAN WEIMER
advertisement

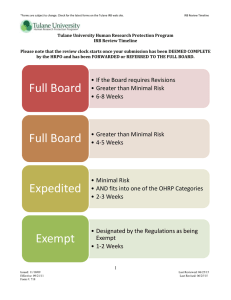
HUMAN SUBJECT RESEARCH COMPLIANCE PRESENTED BY BRIAN WEIMER TULANE UNIVERSITY RESEARCH COMPLIANCE OFFICER July 23, 2013 Objectives of Presentation What is the role of the Research Compliance Officer? What is noncompliance? What should I expect during an audit? Why does it matter if human subjects research is involved? Does my research involve human subjects? Is IRB review of my research required? What are the different levels of IRB Review? Role of Research Compliance Officer Compliance role on the following committees: Institutional Review Board (IRB): http://tulane.edu/asvpr/irb/index.cfm Institutional Animal Care and Use Committee (IACUC): http://tulane.edu/asvpr/iacuc/index.cfm Institutional Biosafety Committee (IBC): http://tulane.edu/asvpr/biosafety/committee/index.cfm Radiation Safety Committee: http://tulane.edu/oehs/radiation/radiationsafety.cfm Conflicts of Interest Committee: http://tulane.edu/counsel/conflict-of-interest-policy.cfm Role of Research Compliance Officer Resource to Tulane community regarding compliance with regulations and university policies that relate to the conduct of research Policy development, training, and auditing Open-door policy for questions from university community Role on IRB: Review proposals for compliance with SOPs and regulations Assist with questions regarding regulations and policies Review and investigate research noncompliance Conducts for-cause and not-for-cause audits Noncompliance Definition: Failure to comply with or adhere to rules, regulations, policies and standards of conduct that govern human subject research; Failure to follow the determinations of the IRB; Failure to follow institutional policies. Noncompliance may be minor or serious, or it may be sporadic or continuing. Serious Noncompliance Noncompliance which increases risks to participants, decreases potential benefits, or compromises the integrity of the human research protection program; generally a reckless or intentional disregard for human subject regulation and policies. Research being conducted without prior IRB approval is always considered serious noncompliance. Continuing noncompliance A pattern of noncompliance that suggests a likelihood that without intervention, instances of noncompliance will continue - three instances of the same type of noncompliance, without adequate explanation, is generally considered continuing noncompliance. Failure to respond to a request to resolve an episode on noncompliance. Noncompliance The IRB makes the determination about whether the compliance is serious or is part of a pattern of noncompliance (continuing noncompliance). The IRB then makes a determination about what action is warranted, including any additional action it deems necessary to protect the rights and welfare of research subjects participating in the study. The IRB must report all determinations of serious or continuing noncompliance to the federal Office for Human Research Protections (OHRP), the FDA (if the study is FDA regulated), and the study’s sponsor (if the project is sponsored) Noncompliance Possible actions: Detail corrective action plan Education/Re-education Modification to the research and/or protocol Increased monitoring Informing study subjects Refer to the Tulane University Research Compliance Officer for investigation and/or audit. Suspension or termination of research Audits – what to expect The Tulane Research Compliance Office conducts not-forcause audits, which means that no specific issue or action prompted the audit, and for-cause audits as directed by the IRB. For-cause audits are occur when the IRB has determined that problems occur on the study necessitating an audit. Not-for-cause audits can be totally random or targeted. Examples of studies where a targeted audit my be appropriate are investigator-initiated studies (due to a lack of a sponsor, as sponsors provide additional oversight), high-risk studies, an inexperienced principal investigator, or a principal investigator with a history of noncompliance. Audits – what to expect Review of Regulatory Binder to ensure that all necessary documents are included and are up to date, including: Current training certificates for research staff Curriculum Vitae for research staff All IRB approved documents, including protocol(s), informed consent documents, and approval letters Delegation of Authority Logs Updated Form 1572 (for FDA regulated studies) Audits – what to expect Review of Regulatory Binder (continued): All correspondence to and from the IRB Protocol Deviation Report(s) and Unanticipated Problem Reports(s) (the auditors will be checking to see that such reports were timely submitted to the IRB in accordance with SOP Sections 8 and 9) All correspondence to and from the sponsor All correspondence resulting from site visit by a monitor (the auditors will be checking that all actions recommended by the site visitors have been completed) Audits – what to expect Review of participant files: Review that informed consent procedures performed correctly If documentation of informed consent was required by the IRB, review that the informed consent documentation was completed correctly and that a copy of the signed informed consent was provided to the participant Documentation that informed consent process was completed prior to any study specific procedures Documentation that participant was provided approved compensation for participation in the research Audits – what to expect Review of drug and/or device accountability logs At the end of the interview, the auditors will conduct and exit interview to review the findings. If you can correct any deficiencies immediately found by the auditors, do so (for example, placing missing items into the regulatory binder). You will then get a letter from the auditors with the findings and required follow-up action items, as well as whether your audit findings will be presented to the convened IRB for further consideration. Audits – what to expect Audit finding are presented to the convened IRB in the case of all for-cause audits and all not-forcause audits if significant and/or numerous issues are found by the auditors. If the audit findings will be presented to the convened IRB, you will receive an IRB action letter regarding whether any further corrective measures are necessary to correct deficiences. Regulation of human subjects research – why is it needed? People imprisoned in Nazi Germany during Holocaust were forced to participate in human experiments – no informed consent. Total deaths uncertain, but death toll was very high. For example, experiments on twins resulted in 1,300 deaths out of 1,500 forced to participate. Other experiments: freezing point of the human body, malaria, mustard gas, sterilization, and effects of high altitude. Led to the passage of the Nuremberg Code: guidance (non-binding) on informed consent, minimization of risk and harm, and the freedom to withdraw. Regulation of human subjects research – why is it needed? Tuskegee Syphilis Study (1932-1972): No informed consent Participants not informed of all known dangers. Participants were never told that Penicillin was an effective treatment for Syphilis, even though Penicillin became widely known as an effective treatment beginning in 1947. Public outcry following an exposé led to the passage in 1974 of the National Research Act, which contained the first mandatory human subject protection provisions. Human Subjects Research Regulations 5 CFR 46: known as the “Common Rule”. Main body of regulations for protection of human subjects participating in research FDA Regulations: 21 CFR 50: protection of human subjects 21 CFR 56: FDA IRB Regulations 21 CFR 312: FDA Investigational Drug Regulations 21 CFR 812: FDA Investigational Device Regulations 45 CFR 164.508-514: HIPAA Regulations State-specific regulations Human Research Protection at Tulane Tulane’s Human Research Protection Program (“HRPP”) includes SOPs that incorporate state and federal regulations, including the “Common Rule”, FDA regulations, state regulations, and the standards of the IRB’s accrediting body, AAHRPP. These regulations and standards: govern all aspects of human subject research protections mandates use of IRB to review research proposals and the composition of the IRB Tulane HRPP SOPs are published at: http://tulane.edu/asvpr/irb/policies.cfm The Tulane Human Research Protection Office (“HRPO”) administers the IRB Role of the IRB The university has charged the IRB with initial and continuing review of all human subjects research occurring under the auspices of the university. In order for investigators at Tulane to receive federal funding, the IRB must file a “FederalWide Assurance” (FWA) stating that the IRB is complying with federal regulations regarding human subjects research and obligating the IRB to enforce human subjects protection regulations. Role of the IRB Investigators are responsible for the initial determination of whether the planned activity comprises human subjects research (Tulane SOPs 3.3). However, the university, as well as sponsors, hold the investigator accountable for wrong determinations. Investigators should err on the side of caution and contact the HRPO (irbmain@tulane.edu) for guidance regarding whether the activity constitutes human subjects research before commencing their research. Role of the IRB Possible Repercussions for failure to obtain IRB approval of human subjects research: Prohibition from using data; Prohibition from publishing; Reporting to federal oversight agencies Subject to filing of a research misconduct allegation, which can lead to a range of penalties, including expulsion. The research misconduct policies are located in the faculty handbook in Part III(H): (http://tulane.edu/provost/upload/Faculty-Handbook2011-12.pdf ) 2-Step process for review of research Step 1: Does the activity constitute human subjects research? Look to prospective intent of the investigator and the definition of research Step 2: If so, does the research qualify for exempt review, expedited review, or convened (i.e., full) board review? Definition of Human Subjects Research (45 CFR 46 & Tulane SOPs 1.4) : Any systematic investigation (including research development, testing, and evaluation) On a living individual about whom the investigation is being conducted Where the investigator obtains Data through intervention or interaction with the individual; or Individually identifiable private information That is designed to develop or contribute to generalizable knowledge Practical Interpretations A “systematic investigation” is an activity that involves a prospective research plan that incorporates data collection, either quantitative or qualitative, and data analysis to answer a research question “Generalizable knowledge” involves studies that are designed to draw general conclusions (i.e., knowledge gained from a study may be applied to populations outside the specific study population), inform policy, or generalize findings Federal regulations do not provide a definition for either “systematic investigation” or “generalizable” knowledge, so decisions are made on a case-by-case basis What is Individually Identifiable Information? (SOP 3.8.5) Information where the identity of the subject is or may be readily ascertained by the investigator, such as: Name Address SSN Phone No. Email address Full Face Photo Finger Print Account Number or any other unique identifying number, characteristic, or code Essential Questions Ask yourself the following: Am I trying to answer a research question? Am I collecting data through intervention or interaction with a living individual or collecting identifiable private information about a living individual? Am I using systematic methods to collect my data? Will I write up the results? Will the results be readily available to others? If yes to all, then the activity likely involves human subjects research subject to review/oversight by Tulane’s IRB Who can serve as Principal Investigator? Faculty, Staff, Residents, and Postdocs Students if a faculty member has agreed to serve as the students’ faculty advisor for submission to IRB It is the faculty advisor’s responsibility to assure that the research is conducted consistent with regulations and Tulane SOPs; and assure closeout of the study CITI training must be taken prior to submission to the IRB CITI Training All associated with Tulane are eligible for CITI Training. Before a submission may be made to the IRB, you must complete CITI Training: https://www.citiprogram.org/ Biomedical researchers should complete the “Biomedical (group 1) Research for Investigators and Key Personnel” module CITI Training modules for responsible animal care and use and financial conflicts of interest are also available. Case Examples Now we will look at examples. As you will see, it is not always a clear cut answer. The answers to each of these questions depends upon the facts and whether the researcher is engaging in a systematic investigation on a living individual about whom the investigation is being conducted where the investigator obtains data through intervention or interaction with the individual or individually identifiable private information that is designed to develop or contribute to generalizable knowledge Is it human subjects research? A study comparing cognitive behavior therapy to medication in treating depression? Yes, this is research, unless the investigator is not doing anything with the data. Focus group study of parenting practices? Maybe. What is the planned use of the data? If it is a systematic investigation and designed for generalizable knowledge, then yes. If presented solely in class, then no. If conducted for quality improvement within the group, then no. Is it human subjects research? Biomedical research on cadavers? No, as this would not involve living human subjects. Study that counts the number of Hispanic surnames in the voter registry in Orleans Parish? No. Counting names in the voter registry is not a “systematic investigation”, i.e., an activity that involves a prospective research plan that incorporates data collection, either quantitative or qualitative, and data analysis to answer a research question. What is not research? Training exercises that do not produce generalizable results Activities designed for educational purposes only Results of class projects that will not be published outside the classroom Examples of publishing outside of the classroom: magazine articles, master’s thesis, doctoral dissertation, poster session, abstract, or any other publication or public presentation. Quality Improvement studies Research involving dead people What is publishing? Look to intent to make the results public. Information used only within a class and not provided outside of the class setting does not constitute publishing. Sharing the information openly with individuals outside the classroom likely constitutes publishing, therefore necessitating review. Is this publishing? As part of a class project, a School of Medicine class collects data from 10 human subjects to answer a research question. The results are not intended to be shared outside the classroom, is this publishing? No If the results are shared via the department’s intranet that is password protected, does this constitute “publishing?” Generally, if the results are confined to limited audience, this is not considered a systematic investigation that is designed to develop or contribute to generalizable knowledge Example: Research Practica Question: A course requires students to undertake projects in which other people are interviewed, observed, or otherwise serve as participants. The purpose of these course projects is to train students and provide them with a closer view of social, educational, or psychological processes, and an opportunity to practice various research methods. Is this human subjects research? Answer: No. The purpose of the activity is to train students to conduct research, not for generalizable knowledge. Research practica are designed to offer students opportunities to learn various research methodologies through practice. Example: Thesis or Dissertation Question: Is research for a thesis or dissertation considered human subjects research? Answer: It depends. A thesis ordinarily involves a systematic investigation that is published or made generally available. The analysis turns on whether data is obtained through intervention or interaction with individuals or whether individually identifiable private information is obtained on a living person. Example: Pilot Studies Question: An investigator plans to undertake a preliminary investigation of the feasibility of a study on a small scale (i.e., fewer than 10 subjects), which will be exploratory in nature. It is designed to help the investigator refine data collection procedures and instruments to prepare a better, more precise research design. Is this research? Answer: No. This study focuses on data collection procedures and research design. This is akin to quality improvement and is not human subject research. However, pilot studies intended to lead to generalizable knowledge are considered human subjects research. Example: Video Interviews Question: Are video recordings of interviews with Hurricane Katrina survivors created to preserve or describe individual experiences to be viewed at a museum considered “human subject research”? Answer: No. The creation of the video recording does NOT intend to draw conclusions, inform policy, or generalize findings. The sole purpose is to create a historical record of specific personal events related to experiencing the hurricane and to provide a venue for survivors to tell their stories. What if the purpose of the interviews is to create an archive for future research? Since the intent is to collect data for future research, IRB review would be needed. Assuming Human Subjects Research Exists, three levels of IRB review Exempt Review; Expedited Review; Full-Board Review (as provided for in federal regulations). Each level requires a different application. Application forms are available on www.IRBNet.org, the IRB’s electronic submission system. If unsure of what level is required for your activity, submit query to irbmain@tulane.edu prior to submitting your application to the IRB. What is exempt research? Minimal risk studies that fall within set categories listed in Tulane SOPs 3.5.2. Shortened application – exempt from further review Exempt studies do not require convened IRB review and are reviewed by the IRB chair (or designee). Exempt research can not include research involving children or prisoners [Tulane SOPs 3.5.1]. Approval period can be for up to three years, unlike expedited or full-board review approval periods, which are for a maximum of one year. Common exempt studies (SOP 3.5.2) 1. Research involving the collection or study of existing data, documents, or records where the sources are publicly available. 2. Research involving the collection or study of existing data, documents, records, pathological specimens, or diagnostic specimens, if these sources are publicly available or if the information is recorded by the Investigator in such a manner that subjects cannot be identified, directly or through identifiers linked to the subjects. Common exempt studies (continued) 3. Research involving the use of educational tests, survey procedures, interview procedures, or observation of public behavior, unless: Information obtained is recorded in such a manner that human subjects can be identified directly or through identifiers linked to the subjects; and Any disclosure of the human subjects responses outside the research could reasonably place the subjects at risk What is expedited research? Research that is no more than minimal risk to subjects and that meets the categories listed in Tulane SOPs 3.5 The identification of participants will not place them at risk of criminal or civil liability or be otherwise damaging Review is conducted by the IRB Chair or the Chair’s designee Approval period is for up to one year Categories of expedited research The research must fit into one of several specific categories to qualify as expedited research (see Tulane SOPs 3.6.1). Common categories include: Collections of blood samples by finger stick, heel stick, ear stick, or venipuncture Prospective collection of biological specimens for research purposes by noninvasive means. Collection of data from voice, video, digital, or image recordings made for research purposes Research on individual or group characteristics or behavior (including research on perception, cognition, motivation, identity, language, cultural beliefs, social behavior, etc.) Categories of expedited research (continued) Clinical studies of Drugs and Medical Devices only when condition (a) or (b) is met. a) b) Research on Drugs for which an IND [21 CFR Part 312] is not required. NOTE: Research on marketed drugs that significantly increases the risks or decreases the acceptability of the risks associated with the use of the product is not eligible for Expedited Review. Research on Medical Devices for which (i) an IDE [21 CFR Part 812] is not required; or (ii) the Medical Device is cleared/approved for marketing and the Medical Device is being used in accordance with its cleared/approved labeling. How long does the IRB take to review a submission? Exempt Review: 1-2 weeks Expedited Review: 2-3 weeks Full Board Review: 4-5 weeks if approved as submitted 6-8 weeks if modifications are required by the board and the PI timely submits the appropriate modifications The IRB approval letter will contain an expiration date for IRB approval of the research. Be sure to submit your continuing review application well in advance of the expiration date. Critical Points to Remember Register with IRBNet in order to submit a protocol to IRB (all submissions are via IRBNet) Complete CITI training prior to submission to IRB. All personnel involved in the project must complete CITI training. Be cognizant of the lead time needed for review by the IRB. Students need a faculty advisor in order to submit a protocol. Do not begin research until you receive the IRB approval letter (MOST IMPORTANT!). Critical Points to Remember (cont.) Be cognizant of expiration dates for approval of the study and for approval of the informed consent document. Most studies are approved for a maximum of one year. A continuing review application must be submitted far enough in advance of the expiration date to allow for IRB review of the continuing review application. File an amendment if any changes are made to the study. If activity originally does not involve human subjects research but your intent changes to include human subjects research, then make a submission to IRB. Contact Information Research Compliance (Brian Weimer): 504.988.1147; bweimer1@tulane.edu; Room 2425 of Tidewater Bldg; http://tulane.edu/asvpr/research-compliance.cfm Tulane’s Human Research Protection Office (HRPO): 504.988.2665; irbmain@tulane.edu; http://tulane.edu/asvpr/irb/index.cfm Tulane human subjects protection policies: http://tulane.edu/asvpr/irb/policies.cfm Instructions for taking CITI Training: https://tulane.edu/asvpr/irb/index.cfm