AN ANALYSIS OF THE EFFICACY OF THE MARINE PROTECTED AREAS IN
MAUI COUNTY IN CONSIDERATION OF TOURISM
Kelly Marie Thomasson
B.A., University of California, Los Angeles, 2002
THESIS
Submitted in partial satisfaction of
the requirements for the degree of
MASTER OF SCIENCE
in
BIOLOGICAL SCIENCES
at
CALIFORNIA STATE UNIVERSITY, SACRAMENTO
SPRING
2011
© 2011
Kelly Marie Thomasson
ALL RIGHTS RESERVED
ii
AN ANALYSIS OF THE EFFICACY OF THE MARINE PROTECTED AREAS IN
MAUI COUNTY IN CONSIDERATION OF TOURISM
A Thesis
by
Kelly Marie Thomasson
Approved by:
__________________________________, Committee Chair
William Avery, Ph.D.
__________________________________, Second Reader
Jamie Kneitel, Ph.D.
__________________________________, Third Reader
Ronald Coleman, Ph.D.
Date: ____________________________
iii
Student: Kelly Marie Thomasson
I certify that this student has met the requirements for format contained in the University
format manual, and that this thesis is suitable for shelving in the Library and credit is to
be awarded for the thesis.
______________________________, Graduate Coordinator __________________
Susanne Lindgren, Ph.D
Date
Department of Biological Sciences
iv
Abstract
of
AN ANALYSIS OF THE EFFICACY OF THE MARINE PROTECTED AREAS IN
MAUI COUNTY IN CONSIDERATION OF TOURISM
by
Kelly Marie Thomasson
The waters of Maui County are essential to the economic livelihood of Maui
County’s residents. In addition to sustenance value, annual revenue from international
tourism and marine activities along the coastlines of Maui County keeps the island's
economy afloat. Ironically, it is this revenue that may lead to the demise of Maui’s coral
reef ecosystems and booming tourism industry. Overuse of Marine Protected Areas
(MPAs), due to their appeal as aesthetically vivid, conservation districts, has potential to
degrade the very systems that the MPAs are intended to protect. Maui Counties MPAs
are exploited as ideal snorkel and dive destinations and potentially used to a higher
degree than non-conserved areas and therefore are more prone to degradation than areas
with no protected status. Marine ecotourism activities have been previously indicated as
factors involved in coral reef degradation.
Here, I attempted to establish whether or not there was a notable decline in
benthic substrate biodiversity in the MPA-managed areas of Maui County relative to that
of non-conserved areas. Based on biodiversity indices, I hypothesized that there would
v
be lower benthic biodiversity at MPA sites (designated as “conserved”) relative to those
sites with no such designations, within the boundaries of Maui County. It was my
prediction that because of the high use of these marine protected areas, relative to control
areas, a more-degraded, less-diverse substrate would be described in the MPAs of Maui
County despite their designation as conservation districts. Photographic quadrats of
benthic organisms and substrate from four tourist-accessible conservation sites and eight
biogeographically similar controls were quantified and assessed through the use of
biodiversity indices at two depth ranges.
Using MANOVAs of Shannon diversity indices, Species Richness and percent
cover, I found significantly lower biodiversity indices in MPAs relative to control areas.
These results were consistent throughout the three assessed biodiversity indices in both
site by site comparisons and an overall comparison of all MPA data points compared to
all control data points. Given the lengthy establishment of MPAs in Maui County and the
importance of marine preservation for the aesthetics, economy and biology of Maui
county, these results suggest the need for change in the level of MPA usage and
regulation of that usage.
_______________________, Committee Chair
William Avery, Ph.D.
vi
ACKNOWLEDGMENTS
I would like to thank my extended family in Maui: Jenny Munday for her support
and generosity, Seager MacDougall, for his technical help with equipment, and
Haley Van Weeman Van Noord, for her encouragement and support. I would also like
to thank my volunteer dive team: Paul W. Skarbo, Jess Rickard, Josh Balthazar, Tia
Rose, Sara Blank, and Kelly Brewer for all their hard work and professional execution. I
would like to extend my deepest gratitude to Teri Leonard and Maui Dreams Dive
Company for their expert advice about dive sites and weather conditions. Finally, I
would like to thank Arthur James Calanchini for offering his expertise in statistics, Alex
Lindsey for his help with digital overlay and data quantification, and the members of my
advisory board: Drs. Bill Avery, Ron Coleman and Jamie Kneitel for their guidance and
support.
This thesis is dedicated to my parents: Gary Lynn and Monta Marie Thomasson
for their unconditional love and support in all my endeavors.
vii
TABLE OF CONTENTS
Page
Acknowledgments............................................................................................................. vii
List of Tables ...................................................................................................................... x
List of Figures .................................................................................................................... xi
INTRODUCTION .............................................................................................................. 1
Maui Ecology ............................................................................................................. 2
Coral Reef Systems of Maui County ......................................................................... 9
Ecotourism and Diver Damage to Coral Reefs ........................................................ 10
Other Physical Stressors to the Coral Reef Community .......................................... 11
The Economy of Maui ............................................................................................. 14
Marine Protected Areas ............................................................................................ 15
Marine Protected Areas in Maui County ................................................................. 17
OBJECTIVES AND HYPOTHESES ............................................................................... 22
MATERIALS AND METHODS ...................................................................................... 24
Data Acquisition ...................................................................................................... 24
Sample Quantification.............................................................................................. 28
Statistical Analysis ................................................................................................... 29
RESULTS ......................................................................................................................... 31
Qualitative Site Observations................................................................................... 31
viii
Tourism Observations .............................................................................................. 31
Descriptive Statistics ................................................................................................ 33
Multivariate Analysis of Variance ........................................................................... 36
Depth Analysis ......................................................................................................... 38
Analyses of Variance by Site Location .................................................................... 44
DISCUSSION ................................................................................................................... 54
Qualitative Site Observations................................................................................... 54
Tourism Observations .............................................................................................. 55
Overall Analysis: Marine Protected Areas compared to control group ................... 56
Site 1 Analysis ......................................................................................................... 58
Site 2 Analysis ......................................................................................................... 60
Site 3 Analysis ......................................................................................................... 61
Site 4 Analysis ......................................................................................................... 63
Depth Analysis ......................................................................................................... 66
Future Studies .......................................................................................................... 67
CONCLUSION ................................................................................................................. 69
LITERATURE CITED ..................................................................................................... 71
ix
LIST OF TABLES
Page
Table 1. Mean tourist counts at each site location and management type. ....................... 32
Table 2. Overall mean descriptive statistics ..................................................................... 35
Table 3. Multivariate Analysis of Variation based on independent variables
and interactions of those variables ..................................................................... 42
Table 4. Wilk's Lambda values for individual independent variables and
interactions of those variables ............................................................................ 42
Table 5. Analysis of percent coral cover by site and overall sites. ................................... 46
Table 6. Analysis of species richness by site and overall sites. ........................................ 49
Table 7. Analysis of Shannon diversity index by site and overall sites. ........................... 51
Table 8. Mean descriptive statistics of all Marine Protected Area and
control sites per site number. .............................................................................. 65
x
LIST OF FIGURES
Page
Figure 1. The islands of Maui County ................................................................................ 4
Figure 2. A map of Maui County with major and minor currents .................................... 13
Figure 3. The tourist accessible Marine Protected Areas of Maui County .......................... 20
Figure 4. Biogeographically similar, control site selection areas and
associated Marine Protected Areas.. ...................................................................... 25
Figure 5. An overall analysis of differences in management by diversity
indices ................................................................................................................. 37
Figure 6. An analysis of differences in management by diversity
indices and depth ................................................................................................ 39
Figure 7. Interactions and variation when separated by depth and site
number ............................................................................................................... 53
xi
1
INTRODUCTION
In today’s ever-changing world, humans strive for progress, development and
technological advancement, often to the detriment of the natural environment and those
species that live within it. In an attempt to prevent progress from destroying the
resources on which it relies, species-based and habitat-based conservation plans have
been incorporated into both U.S. domestic and international law (Bean, 2009). Since
their inception, however, conservation based regulations such as the Endangered Species
Act (ESA) and establishment of Marine Protected Areas (MPAs) have been met with
heated conflict from human stakeholders (Bean, 2009; IUCN, 2010; Stevenson and
Tissot, 2009). In the case of MPAs in Hawai’i, it is unclear whether or not efforts to
appease those stakeholders have gone so far as to nullify the aesthetic and ecological
benefits of protecting those areas.
The tourism industry, according to the Hawai’i's Department of Commerce, is
Hawai’i's primary source of revenue (Hawaii.gov, 2009). Tourism and economic
prosperity are prioritized over purely environmental conservation efforts with the
establishment of minimally restricted, highly accessible MPAs (IUCN, 2010). Areas
designated as “protected” may be subjected to more use and consequential degradation
because of their appeal as pristine or undisturbed regions. Here, I attempt to analyze the
efficacy of the MPAs in one county within Hawai’i, Maui County, in which the primary
economic resource is tourism. The subject of this research is based on three, often
conflicted forces: Maui ecology, Maui economy, and Marine Protected Areas.
2
Maui Ecology
Maui County is a small region within the Hawai’ian island chain, which was
formed by volcanic plumes and their overlapping volcanic flows. The four islands and
one islet within Maui County have no lakes and few rivers or streams (Sinton, 2003).
The County’s islands surround a relatively shallow region of ocean referred to as an
isobath which acts as a seasonal home to Humpback whales and hosts a multitude of
coral reef communities (HDLNR, 2009; Figure 1). The nutrient poor conditions and
clarity of the water are key factors that make coral the primary substrate of this marine
ecosystem (Karleskint, 1998).
Coral reefs are dynamic and species-rich ecosystems which not only serve as a
home for thousands of reef fish, benthic creatures, and the occasional marine mammal,
but also play a vital role as marine nurseries and primary productivity sources (Hubbell,
1997). The key biotic substrates in a coral reef environment are members of the reefbuilding coral group, in the class Anthozoa (Fedorowski, 2009). Reef-building corals are
highly adapted organisms that have evolved to outcompete other organisms in nutrientpoor, tropical regions where waters are warm and sunlight, prolific. Members of the
coralline anthozoans are capable of living in these environments because they have
formed a symbiotic relationship with photosynthetic unicellular algae called
zooxanthellae (Riegl et al., 2009; Manzello, 2010). Because they have adapted to thrive
in such specific conditions, the distribution of these organisms is severely limited by both
biogeographical constraints and the corals’ adapted physiologies. Like those of other
marine species, coral distributions are highly dependent on a balance of physical
3
(Schutter et al., 2009; Zelnio et al., 2009; Molodtsova et al., 2008), chemical (Manzello,
2010) and biological components (Littler et al., 1983; Riegl et al., 2009). When these
components fall out of balance, coral reefs weaken and degrade. In the field of
ecological conservation, these highly specialized coral organisms face higher threats of
endangerment and extinction because of their specialized nature (Mumby and Harborne,
2010).
4
Molokai
Maui
Lana’ i
N
Kahoolawe
Figure 1. The islands of Maui County. Maui County also contains the islet, Molokini,
which is located between the islands of Maui and Kahoolawe.
5
In a pristine coral reef environment, there is some competition between coral and
algae. These algae are kept at low densities by herbivorous consumers and low nutrient
levels (Littler et al., 1983). Algae are capable of living in most systems, but corals have
persisted in their habitats because they are capable of outcompeting algae for space and
resources in habitats with the specific parameters mentioned above. When a coral reef
habitat changes due to thermal stress, loss of herbivorous reef fish or increased nutrient
levels, the algae often overgrow the coral, suffocating and killing it. Thus, the terrestrial
biogeography of the region, specifically the proximity of point-source pollution, can
further limit the distribution of reef-building corals by enhancing the competitive success
of algae (Riegl et al., 2009).
Additional evolutionary and physical factors may alter the competitive success of
algae and coral. Algal resistance to herbivory by fish has been documented in several reef
ecosystems (Littler et al., 1983), while decreases of water flow have shown to allow for
more successful recruitment and propagation of algae over the coral head (Schutter et al.,
2009). Both microevolutionary events and environmental fluctuations overtime are
naturally occurring in these and all systems, so one would also expect to see fluctuations
in biodiversity levels as well as changes in the species composition within a coral reef
community. These fluctuations should be relatively consistent across areas with similar
biogeographical characteristics.
Coral communities are dependent upon many of their inhabitants for regulation of
growth, genetic heterogeneity, and removal of their algal competitors. Parrotfishes and
other reef fishes, are key regulators in coral reef communities. All functioning coral reef
6
communities have parrotfishes or other grazers, which act as multi-factorial regulators
(Hixon and Brostoff, 1985; Mumby, 2006; Howard et al., 2009).
Peter Mumby (2006; Mumby and Harborne, 2010) has found that parrotfishes are
a key species in the regulation of coral reef habitats and the recovery of those habitats
after disturbances such as hurricanes. Parrotfishes both remove algal cover and increase
heterogeneity by removing pieces of coral substrate and allowing space for new polyp
recruitment (Mumby and Harborne, 2010). Removal of patches of coral allows new,
genetically different coral to take up residence in a reef that would otherwise be much
more homogenous, and therefore vulnerable to complete collapse if susceptible to disease
or environmental change. Genetic diversity promotes survival of at least some of the reef
in the case of environmental changes that degrade portions of the reef, and parrotfishes
promote this genetic diversity through grazing (Mumby, 2006). This grazing behavior is
also a necessary factor in the recovery of degraded or damaged coral reefs because it
initiates the normal processes of coral recruitment, attachment and growth (Mumby and
Harborne, 2010).
Parrotfishes (family: Labridae) are a group of fishes related to the wrasse family
and in the order perciformes (Westneat and Alfaro, 2005; Streelman et al., 2002; Ferreira,
2005). Their global distribution is concentrated in the tropical and subtropical regions
and within coral reefs, rocky coastlines and seagrass beds. Parrotfishes have fused,
parrot-like beaks which play a key role in their feeding behaviors which includes picking,
scraping and removing substrate, often coral, in order to acquire food.
7
Parrotfishes are long-time inhabitants of coral reef communities and have coevolved with these reef communities (Byrne, 1970; Videlier et al., 1999; Streelman et al.,
2002). Howard et al. (2009) analyzed the community structure of parrotfishes,
illustrating the changes within the substrate of the community as the composition and
ratio of parrotfish types changed. In coral reefs off the island of Oahu, where excessive
fishing had depleted the number of large parrotfishes and the overall diversity of
parrotfishes, these researchers found decreased substrate rugosity, numbers of benthic
epifauna and percent live coral. This indicates that the presence of parrotfishes and the
diversity of parrotfish species, within a coral reef, are important to the community
structure of that reef (Bellwood and Choat, 1990; Howard et al., 2009). While
parrotfishes populations are regulated in many different ways (Cardwell and Liley, 1991;
Guyton and Hall, 2005), reef robustness, defined as resilience to damage, resistance to
environmental stressors, and rapid successive growth (Mumby, 2006; Weiss, 2010) is one
of the key factors in this regulation. A smaller or less robust reef will support fewer
parrotfish sustainably; therefore parrotfish population size may indicate the robustness of
the reef in question (Mumby and Harborne , 2010).
According to Bellwood and Choat (1990), the morphological and behavioral
differences between two types of parrotfishes, the excavators and the scrapers, were
dissimilar enough in behavioral characteristics including bite speed, niche utilization and
food specificity, to consider them two different groups ecologically. They went on to
explain that this separation is significant when considering the parrotfishes’ role as an
ecosystem regulator. Coral reef ecosystems with parrotfishes of the scraper variety will
8
be subject to different levels of top down regulation (both lower amounts and in different
areas) than those ecosystems with excavating parrotfishes (Bellwood and Choat, 1990;
Streelman et al., 2002). As is often the case in ecological systems, Bellwood and Choat,
suggest a mixture of the two types is ideal for balanced regulation of coral reef
ecosystems (Bellwood and Choat, 1990).
Also key in proliferation and regulation of coral reef systems are the benthic
organisms that live among and within the reefs. The benthic epifaunal composition of a
coral reef community is dependent on the diversity of coral in that reef habitat and how
much shelter it provides or light source, it allows. Variation in substrate is also a factor
in the benthic diversity of any given subsection of a larger reef (McKindsey and Bourget,
2001). Some organisms, like the Petroglyph shrimp (Alpheus deutropus), require a
specific coral in order to create burrows in which to live, while other organisms such as
the spiny oyster (Spondylus nicobaricus) require sandy bottoms for burrowing (Hoover,
1998).
Just as the type of coral in a habitat can determine the other types of benthic
organisms that live there, the benthic creatures can shape the substrate overtime.
Different assemblages of benthic creatures can eventually determine the coralline
substrate in the area (Garcia-Sais, 2010). Sea urchins (class: Echinoidea), for example,
affect the coral substrate on which they live by carving divots in flat coral lobes
(McClanahan and Shafir, 1990). Algal diversity, pH, and salinity affect benthic epifauna,
in addition to temperature, light and coral diversity (Klugh, 1924) and these factors are all
affected by the type of benthic assemblage in a particular coral reef (Garcia-Sais, 2010).
9
Regulation of these factors is neither solely bottom-up or top-down but rather multidirectional and said to regulate the marine system synergistically (Tewfik, 2005); thus,
non-coralline benthic diversity is an indication of coral diversity and vice versa.
Parrotfishes also determine benthic assemblages, through substrate alterations (Brock,
1979). The parrotfishes, benthic epifauna and coral reef substrate all function
symbiotically to keep the system in balance.
Larger organisms also interact with, and thus affect coral reef environments. Sea
turtles act as important regulatory components of this system because they, like many
other organisms, eat algae (Smith et al., 2010) and thus, the presence of large numbers of
turtles may indicate there are larger amounts of available algae (Pacific Whale
Foundation, 2006; Lehman et al., 2006). There are far more direct examples, however, of
the anthropogenic effects on coral reef habitats, which can be observed at the chemical,
physical and biological levels.
Coral Reef Systems of Maui County
Maui County boasts a very high rate of endemism, classifying many of its
terrestrial, insect and bird species as endemic at the family level. About twenty percent
of Hawai’i’s marine invertebrates and twenty-four percent of its fishes are considered
endemic to the Hawai’ian Islands; many of these species live within the islands’
surrounding reefs (Hoover, 1998). Because of the colorful and diverse array of sea
creatures found in Hawai’i, the state boasts an impressive aquarium fishery and marine
ecotourism industry (Stevenson and Tissot, 2009).
10
Ecotourism and Diver Damage to Coral reefs
Studies on other coral reef systems have indicated that tourist impact is significant
(Tratalos and Austin, 2001; Van Treeck and Schumacher, 1998; Hasler and Ott, 2008).
Increased tourism can be correlated with increased snorkelers, divers and swimmers, who
step on coral, kick coral lobes, harvest coral, and scrape coral polyps. In addition to the
deleterious effects of chemical pollutants such as sunscreen (Danovaro et al., 2008;
Burgess, 2006), snorkelers and divers further exacerbate the destruction of this delicate
ecosystem by causing physical damage to reefs in the form of scraping, or breaking of the
coral, whether it is intentional or not (Hasler and Ott, 2008; Barker and Roberts, 2004)
and trophic system interruption due to tourists feeding fish (Burgess, 2006). This
artificial food source disrupts the natural food chain within the system, preventing some
fishes from eating as much of their normal food source. In the case of coral reef fishes,
this may mean lower consumption rates of algae, the corals’ primary competitor in areas
where point source pollution provides new sources of nutrients (Burgess, 2006; Gardner
et al., 2005).
Fin-kick damage is the most egregious offender in terms of physical
anthropogenic damage. A study by Hasler and Ott (2008) provided evidence that
physical damage to corals decreases with increasing distance from the shore.
Furthermore, an increase of shore-vicinity damage was found near sites known as
SCUBA diver entry sites (Hasler and Ott, 2008). While the paper by Hasler and Ott
makes a clear and substantiated point with respect to reef damage by SCUBA divers, it
11
fails to comment on the effects of the larger and potentially more awkward group: the
snorkelers.
Boats and commercial tourism companies are also major contributors to the
deleterious effects that tourists have on coral reef ecosystems. Anchor-dragging damage,
poor mooring placement, and boats running aground are all examples of physical damage
caused by boats (Jameson et al., 1999). Boat companies are the culprits of biologicallybased coral ecosystem damage through the promotion of fish feeding (Pacific Whale
Foundation, 2006), and chemical pollution through oil, gasoline and sewage discharge
(Jameson et al., 1999; Grigg, 1994).
Other Physical Stressors to the Coral Reef Community
Coral reef systems are highly susceptible to outcompetition by their algal
competitors when the systems’ characteristics change (Schutter et al., 2009 and Littler et
al., 1983; Gardner et al., 2005). The addition of pollutants, not only adds chemicals
potentially toxic to the coral polyps or caustic to its CaCO3 skeleton, but is also capable
of reducing water clarity and thus the ability of the photosynthetic zooxanthellae to
photosynthesize. Photosynthesis is a process necessary for corals to acquire carbohydrate
resources and for the survival of the coral colony. Reduction of this process can result in
coral stress, sickness, starvation and bleaching. Coral bleaching is a stress-response of
corals during which, a coral polyp ejects its symbiotic zooxanthellae in an effort to
preserve food resources. Usually coral bleaching is a precursor to coral death because
corals cannot survive for long without the assistance of zooxanthellae to acquire food. If
12
the coral is not bleached due to algal infection, it is often the victim of disease because of
increased susceptibility to viral infection (Riegl et al., 2009).
Tewfik (2005) found that with increased pollution the benthic community food
web shifts from a mix of generalists and specialists to a generalist-dominated community,
with a near-depletion of specialists (Tewfik, 2005). This is of particular concern with
respect to coral reef communities where benthic and nektonic organisms are often highly
specialized and are thus at a higher risk of effects of pollution and ultimate decimation
(MacArthur and Wilson, 1967). Because these pollutants are distributed by currents, it is
important to consider current direction and flow rate when determining the areas that will
be most likely affected by this. Figure 2 illustrates the MPAs of Maui County in relation
to the currents of the area.
13
Molokai
Maui
Lana’ i
Kahoolawe
Figure 2. A map of Maui County with major and minor currents. This map indicates the
four islands within its boundaries, the four accessible Marine Protected Areas, potential
control sites for this study and major and minor currents (in black and dashed lines
respectively). Adapted from Google Maps.
14
Thermal stress is also a significant factor in some of the more-shallow coral
species distributions. Increases in thermal waste point source pollutants, paired with
overall oceanic temperature increase (despite its seemingly small overall change) lead to
coral bleaching, coral death or degradation (Munday et al., 2009). In a study by
Kuwahara et al., (2010), UV radiation levels were found to be a significant factor in the
efficiency of photosynthesis and the thermal stress which is incurred by both the coral
polyp and its symbiotic zooxanthellae (Kuwahara et al., 2010). Some corals have
adapted to this thermal stress by taking in zooxanthellae that are thermally tolerant
(referred to as “D class zooxanthellae”) These adapted zooxanthellae are often found in
regions most affected by higher UV radiation levels but have been correlated with lower
rates of reef formation (Weis, 2010). Additionally, ocean acidification is a secondary
result of this increased UV radiation, which leads to chemical degradation or dissolution
(Fedorowski, 2009). Despite the presence of thermally tolerant zooxanthellae, the high
UV radiation levels at the equator will cause an increase in water temperature, and
consequently higher rates of evaporation, leading to ocean acidification which causes the
coral skeleton to dissolve (Manzello, 2010). Therefore, despite biological adaptations,
the physical and chemical properties of coral ecosystems are still vulnerable in this
rapidly changing environment.
The Economy of Maui
Currently, tourism is Maui County’s primary revenue source (Sinton, 2003;
Hawaii.gov, 2010). While many people come to Maui to relax and be pampered; the
15
ocean is a major source of the activities available to tourists. Daily, boats take thousands
of people to Molokini crater, Lana’ i and other popular scuba and snorkel destinations. In
the winter months, whale watching trips and surfing events are an added source of
revenue. Cruise ships from several cruise services also use Kahului and Lahaina, both on
the island of Maui, as a port of call (Pacific Whale Foundation, 2006). With the
expansion of tourism came an increase in permanent Maui County residents (Sinton,
2003). This increase in population has been correlated with an increase in algal
recruitment near point source pollution points on the islands (Pacific Whale Foundation,
2006; Smith et al., 2010). Recruitment of algae places algae and coral in direct
competition with each other for space, and as the waters around Maui County become
more polluted, the algae gain the adaptive advantage (Karleskint, 1998; Schutter et al.,
2009 and Littler et al., 1983; Gardner et al., 2005).
Marine Protected Areas
The concept of the MPA was first established in 1990 by the IUCN under the
leadership of Sylvia Earle. The new concept of the Marine Protection Area network was
to have a global record of the conserved areas so that policy makers and scientists could
work together to effectively conserve oceanic resources (Almany et al., 2009; Ban et al.,
2009; MPAC, 2009; IUCN, 2009). Marine protected areas serve several functions. They
act as ecosystem-wide, multi-use bioregions of regulated recreation and minimal take
(Harding et al., 2001). While the most commonly assumed function is preservation of
the designated ecosystem, MPAs also act as sources of larval recruits or seed banks, as
16
well as nurseries and indicators of problems for the larger ecological system (O’Connor
et al., 2007; Mumby, 2006; Mora et al., 2006). One of the largest departures from
typical, terrestrial-based conservation movements is that of MPA planning and
designation. Unlike conservation areas designated prior to MPAs (Bean, 2009), these
areas are established by many stake holders including scientists, government officials and
local community members. The intent is to protect areas of vital importance to the local
marine ecosystem, without debilitating commerce and community (Agardy, 1994;
Fearon, 2003; Ban et al., 2009).
Each MPA is established based on its determined ecological importance to the
stability of the surrounding system. The designating board also considers the economic
or commercial importance of this area when determining the level of use allowed within
its boundaries (Ban et al., 2009). Many MPAs have zonation within the designated
borders, providing commerce and recreation with a larger use permit in the exterior
zones. Also important is the proximity of MPAs to other MPAs; creating an MPA
network is vital to the MPA’s overall efficacy (IUCN, 2009; NOAA, 2010; Mora et al.,
2006; Planes et al., 2009). Despite their scientific design, even large MPAs are incapable
of encompassing every possible organism with which that ecosystem interacts. It is for
this reason that multiple MPAs are necessary within close enough proximity that the sites
can exchange species (Almany et al., 2009). Increased numbers of MPAs within an area
increases the probability that most species will be protected within at least one site
(Planes et al., 2009).
17
In 1999, nine new MPAs were designated along the west coast of the big island,
Hawai’i, an area that is host to the biggest aquarium fishery in the state. The designation
of these stringent, no-take MPA networks effectively eliminated over a third (35%) of the
available fishery. In early 2009, a survey was conducted by the IUCN to determine
sentiment and public opinion of MPA success rate, based on the socioeconomic status of
the fishermen around those waters. Most aquarium fishermen working off the west coast
of Hawaii reported an increase in both number and quality of fish caught in the remaining
65% of coast line. Most also reported some improvement in health, finances and other
socioeconomic criteria (Stevenson and Tissot, 2009). From this perspective, the MPAs
seem capable of performing the task for which they have been established.
Although MPAs are expected to act as conservation and sourcing sites for species
within the area’s boundaries, many factors, such as surrounding degraded or polluted
ecosystems, poorly reinforced MPA site regulations, or inadequate MPA zonations, can
impede progress towards the intended goal. Furthermore, in areas of high use due to
ecotourism, accessible MPAs attract a multitude of snorkelers and divers because of their
protected status, thereby degrading the protected area through over-use, potentially more
so than the neighboring, unprotected areas.
Marine Protected Areas in Maui County
There are approximately five thousand registered Marine Protected Areas within
the Marine Protected Area Network; two hundred twenty-five of these are U.S managed.
The largest Marine Protected Area to date is part of the Hawai’ian Archipelago.
18
Established in 2006 by President George W. Bush, the Northwestern Hawai’ian Islands
Marine National Monument, now called Papahānaumokuākea, is 362,600 km2. There are
twenty-two MPAs in the state of Hawai’i and six in the county of Maui (IUCN, 2009).
Around the islands of Maui, Lana’ i, Molokai and Kahoolawe, locally known as the
collective Maui Nui and designated by the state as Maui County, there is little evidence
of MPA success as little research has been published to determine the functionality of the
local MPAs (KIRC, 2009). These MPAs also differ significantly from others within the
Hawai’ian archipelago in that they are both accessible to tourists and used almost daily
by tourists, year round.
Ahihi-Kinau Marine Preserve and La Perouse (MPA site 1; Figure 3) are on the
southeastern coast of Maui. This MPA was established as Maui County’s first Marine
Conservation district in 1973. Access is by land or boat and is bordered by private
residences and lava fields. This MPA includes land in its protected boundaries; its area is
3.26 km2 of sea and 8.27 km2 including land (IUCN, 2009). This area is a popular diving
and snorkeling site where recently, evidence of ecotourism damage has recently been
addressed when a popular snorkeling area known as the “fish-bowl” or the “aquarium”
was closed due to habitat deterioration (HDLNR, 2010).
One of Maui County’s most famous snorkeling sites, Molokini (MPA site 2;
Figure 3) became its second marine conservation district in 1977. It is approximately
0.31 km2 in size and is one of three submerged caldera globally. It is a crescent shaped
islet located in the `Alalakeiki Channel about 3 miles off Maui's southwestern coast.
Access is by boat only, and there are no facilities on or access to the island itself as it is a
19
seabird sanctuary and rookery for great frigate birds or Iwa (HDLNR, 2010; IUCN,
2009). Molokini too, has been the recent target of ecological controversy and media
attention because of its daily use by snorkeling and diving companies (Maui News,
2010).
Honolua-Mokule`ia Bay (MPA site 3; Figure 3) was established as Maui
County’s fourth Marine conservation district in 1978. Honolua Bay is located on the
northwestern coast of Maui. Mokule`ia Bay is southwest of, and adjacent to, Honolua.
The total MPA is approximately 0.18 km2. In spring, summer and fall, this is a popular
snorkeling destination for tourists, but can have murky water conditions due to run off
from location resorts and golf courses (IUCN, 2009). In winter, Honolua bay is host to
impressive wave action and consequently several international surf competitions
(HDLNR, 2010; Save Honolua, 2009).
Manele and Hulopo`e Bays (MPA site 4; Figure 3), on Lana’ i, were established
as Maui County’s second Marine conservation district in 1976. Totaling 1.25 km2, this
MPA is composed of two adjacent bays on the southern coast of Lana’ i (IUCN, 2009).
It is accessible by boat or by land and has significantly fewer visitors to its waters than
other Maui county MPAs as access to the island itself is cost prohibitive (Pacific Whale
Foundation, 2006). This MPA has different zoning levels for each bay; Hulopo’e bay
prohibits all boat or watercraft access, but allows snorkeling and diving, while Manele
bay permits boat access as well as snorkeling and diving (IUCN, 2010). Manele Bay is
also a major harbor for the island of Lana’ i; it experiences small boat traffic throughout
the day, including a ferry from Maui, which runs five round trips to and from Maui daily.
20
Figure 3. The tourist accessible Marine Protected Areas of Maui County. Above are the
four tested sites: Ahihi-Kinau and La Perouse (A), Molokini (B) Mokuleia and Honolua
(C) and Manele and Hulopo’e (D). Also indicated are the relative positions of these sites
(A,B,C,D) within the boundaries of Maui County (lower map). Adapted from Google
Maps.
21
Despite evidence of the positive effects of MPAs on the island of Hawaii, Maui
has little published evidence of any effects of its six MPAs. Furthermore, with excessive
use that is potentially more than that of the un-protected areas, the four tourist-accessible
MPAs of Maui are subject to a large amount of potential degradation and thus, may not
only be ineffective as source populations and reserves but may demonstrate a more
degraded coralline environment than the un-protected areas. This study will examine the
four tourist-accessible MPAs of Maui County to determine overall ecosystem benthic
biodiversity relative to un-protected areas in an effort to determine whether this is the
case in Maui County. Figure 3 illustrates the location of the four accessible MPA sites in
Maui County.
22
OBJECTIVES AND HYPOTHESES
The objective of this study was to determine the efficacy of the Marine Protected
Areas in place in Maui County as well as to assess the potential negative impact of an
MPA’s attractiveness as a recreation site, towards its goal of increased biodiversity.
Assessment of these MPAs could indicate the potential need for rezoning or revision of
the current MPA boundaries or regulations based on levels of ecotourism use for that site.
This study may be influential in revising establishment and management procedures for
future MPAs where tourism is abundant and essential to the region’s economic survival.
Based on biodiversity indices, I hypothesized that there would be lower benthic
biodiversity at marine sites designated as “conserved” relative to those sites with no such
designations (MPA management vs. control), within the boundaries of Maui County. Not
only should this be evident when comparing the collective set of MPAs against the
similar control site collective, but there should also be a clear disparity at the site by site
level, if the MPA designation is the causative variable. As previously described, regular
use of areas within a coral reef ecosystem by ecotourists has been shown to result in
degradation of the system in many ways. It was my prediction that because of the high
use of these marine protected areas, relative to control areas, a more degraded, less
diverse substrate would be described in the MPAs of Maui County despite their
designation as a conservation district. Additionally, I predicted significantly lower
diversity in both of two depth ranges, as both depths (“shallow”: 7 meters and “deep”: 12
meters) are adversely affected by ecotourism within the MPA. The shallow regions are
23
affected by snorkelers and shore-entry divers, while the deeper areas are affected by both
boating and diving activities.
24
MATERIALS AND METHODS
Data Acquisition
There are four tourist-accessible MPAs in Maui County. These sites were
assigned numerical names (MPA 1, MPA 2, MPA 3 and MPA 4) and a list of control
sites for each of these MPAs was established based on tourism maps and biogeographical
data. Non-MPA managed (control) sites were randomly chosen by random number
generator from this predetermined set of publicly accessible sites. When a site was
compromised or inaccessible, the next site was randomly selected from the randomly
ordered list (Figure 4).
At each site, qualitative observations were made regarding fish abundance and
size, as well as the presence of other marine fauna. Human use was also observed by
counting people using each site, both on the beach and in the water (including watercraft
within site boundaries). These counts were performed by a research assistant on the
beach, during the underwater data collection timeframe. In order to determine whether or
not there was a significant difference in benthic biodiversity based on Management
(MPA or control), a set of photographs of benthic organisms and substrate from both
MPA sites and control sites was quantified and compared on several levels. The
photographs were all obtained within the same season as each other in order to rule out
differences in biodiversity due to seasonal changes and annual fluctuations (Smith et al.,
2010). The substrate (rock, coral, sand, etc.) was photographed during the months of
May and June, 2010, at twelve sites: four MPA sites and eight control sites (Mumby,
2010).
25
Molokai
3
Maui
Lana’ i
4
2
N
Kahoolawe
1
Figure 4. Biogeographically similar control site selection areas and associated Marine
Protected Areas. A map of the four tourist-accessible Marine Protected Areas of Maui
County (numbered) and their corresponding areas of biogeographically similar character
(boxed). Paired control sites for each Marine Protected Area were chosen from within
each of these areas.
26
At each site, two depths were sampled: a Shallow depth of 7 meters (acceptable
range was 5-9 meters in depth) and a Deep depth of 12 meters (acceptable range was1014 meters in depth; Mumby, 2010). Within each depth range, a minimum of three
transect lines, each with a randomly selected directional bearing, were laid out from
randomly selected coordinates within that site. Random coordinates and bearings were
selected by assigning numbers to coordinates and bearings and then by use of a random
number generator. Coordinates were generated in a list for each site, such that if the
desired depth range was not achievable at the randomized coordinate, the new coordinate
became the point at which acceptable depth was achieved by swimming due south or
east, depending which bearing is directly away from shore.
The substrate was photographed in ten stratified, random locations as
photographic 0.5 m2 quadrats along each 30 meter transect line. The substrate was
photographed every 3 meters, starting at 3 meters (Krebs, 1999). The sampling locations
(right or left of the transect line) were predetermined based on coin flipping (heads was
right of transect line; tails was left of transect line). If the right or left location fell out of
the accepted depth range, the side was switched. If neither the right nor the left sample
was within depth range, the point was skipped and the transect was extended 3 meters to
reclaim the point lost on the initial transect. If samples continued to fall out of the
accepted depth range more than three times in a row on the transect, or if the transect ran
into barrier, the transect line was turned 90 degrees clockwise from the point of the last
photograph until the problem was corrected, and the remaining points were photographed
at the new bearing.
27
Quadrats were photographed with a Sea and Sea DX 1200 HD underwater digital
camera with a multi-lumen range YS27-DH strobe and additional 30 lumen floodlights.
To ensure a similar-size photograph area or a normalized photographic quadrat, the
camera was calibrated at a specific zoom level and maintained at a specific distance from
the substrate, using an attached distancing rod (Avery, 1998). A 0.5 m2 quadrat was laid
down to indicate borders of photographic quadrat area while calibrating zoom. If any
photograph was indecipherable, that photograph (data point) was thrown out and reobtained. If necessary, the transect line was moved (rotated) 90 degrees as described
above to correct persistent problems with photograph clarity.
Photographic sampling took place between the hours of 8 a.m. and 5 p.m. All
sampling times were recorded to account for the potential that diurnal changes in species
abundance and species richness were a factor in experimental results. If the quadrats
could not be photographed at or around designated times due to hazardous conditions or
regulation (e.g. Marine Mammal Protection Act) postponement, a secondary site was
selected from the most adjacent sites available and the remaining points were
photographed from that site at both depths.
All control sites were biogeographically similar to and within a 3 km distance
from their paired MPA sites. Furthermore, although currents were relatively insignificant
in the areas of focus, I attempted to alternate control sites between above and below
“conserved sites” with respect to current in order to account for potential bias of results
based on current movement. All sites were tourist accessible.
28
Sample Quantification
Shannon diversity, species richness and percent cover indices were calculated
based on analysis and quantification of photographic quadrats. All indices were the result
of 30-50 samples per depth per site and were treated as independent samples due to intersampling distance and total size of the area of interest. Shannon diversity indices (H′)
were calculated by the equation H′ = ∑Si=1 pi lnpi where pi is the proportion of the total
represented species represented by each species, or relative abundance (Krebs, 1999).
For aggregate or clonal species, such as sponges, bryozoans, and corals, specimens were
counted as individuals when they exhibited distinct boundaries separating them from
other specimens of similar or different character (Hixon and Brostoff, 1985). Species
richness index was calculated by counting the number of species per photograph post
dive, and identifying non-coralline, benthic organisms to genus or species. Coral species
were identified to genus as determination of coral species is still a topic of debate (Pacific
Whale Foundation, 2006).
Percent cover indices were calculated by photographic overlay of normal distance
photoquadrat onto a 100 square grid work. Total live coral cover was then estimated at
0%, 25%, 50%, 75% or 100% of one grid square for ten stratified random squares. For
each site, the ten stratified random squares were selected by pairing each row of ten total
rows per grid with a column selected by a 1-10 random number generator.
29
Statistical Analysis
In order to determine whether or not significant differences in the means of
dependent variables (Shannon diversity, species richness and percent cover) existed, a
Multivariate Analysis of Variance (MANOVA) was performed with sites 1-4 on the four
independent variables within each site: MPA designation, no protection status (control),
“shallow” depths (meters) and “deep” depths (meters). Any evidence of variable
interaction or type I error factors was further analyzed using Bonferroni-corrected, post
hoc tests. Additional comparisons were made using statistical Analysis of Variance
(ANOVA) tests for each dependent variable at each site in order to assess potential
differences in the biodiversity indices at each site and to substantiate evidence illustrated
by the MANOVA. Finally, an assessment of depth variability was made using ANOVAs
for depth and management variables on a site per site basis. All analyses were set at an
alpha value of 0.05.
Samples were first treated as independent per site and then the shallow depths
were averaged and reanalyzed for significant differences in biodiversity indices for the
shallow depths only. An average analysis of the deep depths was not performed because
MPA 4 reef habitat samples within the desired “deep” depth range could not be obtained
and absence of these data points reduced the averaged sample set to 3, rendering the
statistical analysis invalid. Additionally, individual ANOVAs were conducted on
individual subsets of each site (i.e. shallow MPA 1 vs. shallow control 1) using
Bonferroni-corrected alpha values of 0.05. To account for the potential of site-4-based
30
error, all analyses of the two management sites as a whole were assessed again, using
sites 1-3 only (omitting site 4).
31
RESULTS
Qualitative Site Observations
Upon review of my field notes and site observations, I found the overall state of
the control sites (un-protected areas) to be more colorful, have more visible benthic
diversity and harbor larger numbers of both adult and juvenile fish. In contrast, the MPA
sites displayed mostly adult parrotfishes but in small number, larger numbers of bivalves,
and substantial amounts of damaged or dead coral debris.
Tourism Observations
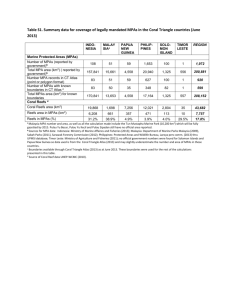
Based on 2-3 site totals throughout the time at each entry site, the research team
counted 50% more tourists at MPA sites than at selected control sites over all (MPA
total=618.75; control total=422.75) with larger relative overall counts on the south
shores of Maui (sites 1 and 2) in both MPAs and controls. All MPA sites experienced
higher rates of tourism based on tourist counts with the exception of site 1 where there
was an average of five times as many tourists at the control sites (Table 1).
32
Table 1: Mean tourist counts at each site location and management type.
Site Management Type
Mean Number of
and Location
Tourists 1
1
MPA 1
Control 1
23.6
100.5
MPA 2
Control 2
236.7
3
MPA 3
Control 3
99
MPA 4
Control 4
86
44.7
per time spent at site
123.3
50
33
Descriptive Statistics
Initial descriptive statistical results indicated larger mean values for the
biodiversity indices of percent cover, species richness and Shannon diversity indices at
control sites relative to those at MPA sites. This was true for both shallow and deep
depths across all three of the selected biological indices (Shannon diversity, species
richness and percent cover, mentioned above).
Mean percent cover values showed a range of 25%-44% percent cover with the
lowest percent cover found in the shallow MPA site average (25% average) and the
highest percent cover found in the deep control site average (44% average). A
comparison of percent cover between MPA site averages and control site averages per
depth showed a nearly two-fold increase in percent cover in the control site groups
relative to the MPA site groups. Of the MPA samples taken, 50 (30% percent of total)
showed 0% cover due to sand channels between reefs or reef death, or erosion. These 0%
cover sites were notable in MPA site 3 in both the shallow depth (Honolua) which has
had past-documented damage from development and recreation, and in the deep depth
(Mokuleia) which has no prior-known significant damage.
Mean species richness values ranged from 7.3-11 with the lowest species richness
value described in shallow MPA group average (7.3 average) and the largest value in the
deep control group average (11 average). MPA sites 1 and 4 can attribute much of its
species richness to bivalve diversity. This was an unexpected and perhaps telling
observation in these two protected sites. In areas where one would expect to find
coralline substrate and benthic epifauna, I found sand, rock, coral debris and filter-
34
feeding organisms. These organisms, although often covered by a light dusting of sand,
are easily identifiable by shell shape, color and growth etchings (Hoover, 1993); thus, I
was able to determine some level of biodiversity within beds of bivalves. The magnitude
of bivalve diversity or number was not evident in any control site, although there were
some bivalves present in most control sites.
The Shannon diversity index values also illustrated a clear difference between the
MPA and control sites. There was an overall value range of 1.4-2.1 with the lowest
value, again, in the shallow MPA group average (1.4 average) and the highest value in
the deep control group average (2.1 average). Table 2 illustrates the average Shannon
diversity, species richness, and percent coral cover values with their corresponding
standard deviations (Table 2).
35
Table 2. Overall mean descriptive statistics.
Site Type by
Mean Percent
Depth and
Coral Cover
Management
1
Mean Species
Richness
Mean Shannon
Diversity Index
Shallow MPA
25%
(0.24)1
7.3
(3.6)
1.43
(0.68)
Shallow Control
44%
(0.29)
9.2
(3.9)
1.81
(0.60)
Deep MPA
27%
(0.28)
7.6
(3.0)
1.64
(0.71)
Deep Control
44%
(0.26)
11
(3.3)
2.07
(0.40)
( ), Standard deviation
36
Multivariate Analysis of Variance
Statistical analysis by MANOVA found the above differences between the two
management types (MPA and control) and the evidence of main effect on biodiversity
indices (Shannon diversity, species richness and percent cover) based on management to
be significant when treating all samples as independent (percent cover p<0.01, species
richness p<0.01, Shannon diversity p<0.01) (Table 2; Figure 5). These differences based
on management (MPA and control) are illustrated in Figure 5.
Additionally, the MANOVA described a significant difference between site
locations exclusively (1,2,3,4) with respect to percent cover, species richness and
Shannon diversity indices (percent cover p<0.01, species richness p<0.01, Shannon
diversity p<0.01) and a significant difference between depths exclusively (shallow and
deep ranges) in for species richness and Shannon diversity indices only (species richness
p<0.01, Shannon diversity p<0.01). The observed change in percent cover exclusively
based on depth, was found to be insignificant based on variability and size of the data set
(p=0.773).
37
A
B
C
Figure 5. An overall analysis of differences in management by diversity indices.
Percent coral cover (A), species richness (B) and Shannon diversity (C) values represent
means of all data points. Error bars represent 95% confidence intervals.
38
Depth Analysis
Considering depth as a variable and potential source of overall effect artifact, the
two management groups were separated by depth, the MANOVA analysis was repeated
and results, plotted. Again the statistics indicated a significant difference in all three
biological indices (Shannon diversity, species richness and percent cover; Figure 6)
between the MPAs and the control sites while the two depth lines supported the overall,
management-based main effect.
39
A
B
C
Figure 6. An analysis of differences in management by diversity indices and depth.
Percent coral cover (A), species richness (B) and Shannon diversity (C) values represent
means of all data points. Error bars represent 95% confidence intervals.
40
Wilk's Lambda multivariate analysis further substantiated these findings and
indicated interactions between the site variables with all F values significantly greater
than their F critical values. The site management variable showed significant differences
for percent cover (p<0.01), Shannon diversity (p<0.01) and species richness (p<0.01)
with a Wilk's Lambda value of 0.811. The site depth variable had a Wilk's Lambda value
of 0.956 and both Shannon diversity indices and species richness were significantly
different (p=0.02 and p<0.01, respectively) but percent cover was not (p=0.773). Despite
the lack of significant difference in percent cover with respect to depth, there was still a
significant difference overall all considering depth as a variable. The site location
variable also yielded significant differences in all three biodiversity indices (Shannon
diversity, species richness and percent cover; all p<0.01) with a lower Wilk’s Lambda
value of 0.660 (p<0.01).
There were also significant interactions between variables. There was an
interaction between site management and site depth variables (Wilk’s Lambda=0.982,
p=0.020) however, only species richness yielded significant results with a p value of
0.049 (percent cover p=0.359, Shannon diversity p=0.706). The three-way interaction
between the three variables of site depth, location and management showed a significant
interaction (Wilk’s Lambda=0.972; p=0.019) but only with regard to percent cover
(p<0.01; Shannon diversity p=0.996; species richness p=0.60). Both interactions
between site management and location variables and between site location and depth
variables were found to be significant (p<0.01) with differences in all three biodiversity
41
indices (percent cover p<0.01; Shannon diversity p<0.01; species richness p<0.01). The
results of this analysis are listed in Tables 3 and 4.
42
Table 3. Multivariate Analysis of Variation based on independent variables and
interactions of those variables.
1
Source of
Variation
Dependent
Variable
Sum of
Squares
F value
P Value
Site Management
Percent Cover
Shannon Diversity
Species Richness
3.53
19.0
768
81
58
76
<0.01
<0.01
<0.01
Site Depth
Percent Cover
Shannon Diversity
Species Richness
.004
7.23
196
.08
22
19
.773
<0.01
<0.01
Site Location
Percent Cover
Shannon Diversity
Species Richness
10.3
11.4
772
79
12
26
<0.01
<0.01
<0.01
Site Management
* Site Depth
Percent Cover
Shannon Diversity
Species Richness
.037
.046
39.1
.84
.14
3.9
.359
.706
.049
Site Management
* Site Location
Percent Cover
Shannon Diversity
Species Richness
3.48
4.08
280
26
4.2
9.3
<0.01
<0.01
<0.01
Site Depth *
Site Location
Percent Cover
Shannon Diversity
Species Richness
.619
4.94
238
4.7
5.1
8.0
<0.01
<0.01
<0.01
Site Management
* Site Depth *
Site Location
Percent Cover
Shannon Diversity
Species Richness
.634
.002
10.2
7.2
.00
.51
<0.01
.996
.600
*, interaction between variables noted
Type III
3
F values are based on a ratio of the differences between groups and the differences within groups
4
Alpha values set at 0.05
2
43
Table 4. Wilk's Lambda values for individual independent variables and interactions of
those variables.
Wilk’s
Source of
Lambda
F
Degrees of
Variation1
Value
value2
Freedom
P Value3
1
Site Management
Site Depth
Site Location
.81
.96
.66
41
8.2
27
531
531
1292
<0.01
<0.01
<0.01
Site Management *
Site Depth
.98
3.3
531
.020
Site Management *
Site Location
.84
11
1292
<0.01
Site Depth *
Site Location
.94
3.6
1292
<0.01
Site Management *
Site Depth *
Site Location
.97
2.5
1062
.019
*, interaction between variables noted
F values are based on a ratio of the differences between groups and the differences within groups
3
Alpha values set at 0.05
2
44
Analyses of Variance by Site Location
In order to further validate these findings based on the individual site pairs, a
series of ANOVAs was run on the site pairs at similar depths. This was done in order to
determine if there was a specific site that acted as a primary contributor to the lower
biodiversity indices found in the MPA sites. Bonferroni-corrected, individual ANOVAS
of the depth subsets and totals for each site indicated differences in biodiversity indices
(Shannon diversity, species richness and percent cover) between different management
types. MPA site 1 showed a percent cover of 34.4%.overall with a shallow percent cover
of 26.4% and a deep percent cover of 45.9%. Control site 1 showed an overall percent
cover of 28.1% with a shallow percent cover of 30.8% and a deep percent cover of
25.3%. The difference between these sites overall was not shown to be significantly
different; however, while there was no significant difference between the shallow sites,
the MPA had a significantly higher percent cover than its control in the deep sites
(p<0.01).
MPA site 2 showed an overall percent cover of 36.4% with values of 42.2% in the
shallow and 30.9% in the deep. Control site 2 values were 60.8% overall with 64.9% in
the shallow areas and 57.7% in the deep. The difference between the sites overall was
found to be significant (p<0.01) as well as the differences in the shallow and deep depths
within that site (both p values<0.01). Site 2 illustrated clear, significant and lower values
for the MPA relative to the control sites.
Site 3 also showed significantly lower percent cover values in MPA areas relative
to the control area both overall (4.2% to 22.0%; p<0.01) and at shallow (4.0% to 17.2%;
45
p<0.01) and deep depths (4.3% to 27.4%; p<0.01). Site 3 showed at least a 4 fold
increase from MPA to control at all levels of analysis.
Site 4 was only analyzed in the shallow samples pair as a deep sample-set for site
4 did not exist. Considering this factor, the total value for the MPA site (22.6%) is the
same as the shallow value 22.6%. The total value for the control site (64.5%) is based on
both available shallow (64.8%) and deep (62.2%) values. The values for both MPA and
control were significantly different from each other in the site total set (p<0.01) as well as
the shallow depth set (p<0.01). These values are listed with their relative comparisons
and p values in Table 5.
46
Table 5. Analysis of percent coral cover by site and overall sites.
Percent
Percent
MPA vs. Control
Coral Cover
Coral Cover
MPA relative
by Site Number
MPA
Control
to Control2
1
All Sites1
26%
44%
Lower**
< 0.01†
Site 1 Total
Shallow
Deep
34%
26%
46%
28%
31%
25%
Higher
Lower
Higher*
0.11
0.41
< 0.01†
Site 2 Total
Shallow
Deep
36%
42%
31%
61%
65%
58%
Lower*
Lower*
Lower*
< 0.01†
< 0.01†
< 0.01†
Site 3 Total
Shallow
Deep
4.2%
4.0%
4.3%
22%
17%
27%
Lower*
Lower*
Lower*
< 0.01†
< 0.01†
< 0.01†
Site 4 Total
Shallow
Deep
22%
22%
-----
63%
65%
62%
Lower*
Lower*
-------
< 0.01†
< 0.01†
-------
All Sites was analyzed with and without site 4 and p values were the same for both.
*, indicates significant difference between sites at α=0.05.
3
†, indicates difference was significant after Bonferroni correction.
2
P Value3
47
With respect to species richness, MPA site 1 yielded an overall value of 8.0 with a
shallow depth value of 7.2 and a deep depth value of 9.1. The control for site 1 yielded a
species richness value of 9.3 overall with a shallow species richness value of 8.2 and a
deep value of 10.4. The overall p values for MPA site 1 and control site 1were found to
be significantly different from each other (p<0.01) but individual subset values based on
depth, although different in number, are not significantly different (shallow p=0.22; deep
p=0.10).
Species richness values for MPA site 2 are 7.2 overall with a shallow depth value
of 7.6 and a deep depth value of 6.7. Control site 2 yielded an overall species richness of
11.7 with subset values of 11.4 and 11.9 for shallow and deep depths, respectively. The
differences between the MPA and control areas for site 2 were found to be significant
overall (p<0.01) and at shallow (p<0.01) and deep (p<0.01) depths. Control site 2 showed
a species richness value that was 50% higher than that of MPA 2 at each level of analysis.
MPA site 3 yielded an overall species richness value of 6.2 with a shallow species
richness value of 5.1 and a deep value of 7.2. The control for site 2 had an overall
species richness value of 7.5 with shallow and deep values of 5.6 and 9.5, respectively.
However, while all three values were lower in the MPA sites relative to their controls,
only the deep depth difference between MPA 3 and control 3 was considered significant
(deep p<0.01; shallow p=0.54; overall p=0.06).
Site 4 showed an overall species richness value for the MPA of 8.9 which was the
same as, and based solely on the shallow value (8.9) as there was no deep data set for
MPA site 4. control site 4 showed an overall species richness value of 11.9 with shallow
48
and deep values of 11.8 and 12.0, respectively. Comparison of the overall values for
MPA site 4 yielded a significant difference between the values (p<0.01), and this was
true for the comparison between the shallow depths of MPA 4 and control 4. These
values are listed with their relative comparisons and p values in Table 6.
49
Table 6: Analysis of species richness by site and overall sites.
MPA vs.
Species
Species
Control by Site
Richness
Richness
MPA relative
Number
MPA
Control
to Control2
All Sites1
7.4
10
Lower*
1
Site 1 Total
Shallow
Deep
8.0
7.2
9.1
9.3
8.2
10
Lower*
Lower
Lower
< 0.01†
0.22
0.10
Site 2 Total
Shallow
Deep
7.2
7.6
6.7
12
11
12
Lower*
Lower*
Lower*
< 0.01†
< 0.01†
< 0.01†
Site 3 Total
Shallow
Deep
6.2
5.1
7.2
7.4
5.6
9.5
Lower
Lower
Lower*
0.06
0.54
< 0.01†
Site 4 Total
Shallow
Deep
8.9
8.9
-----
12
12
12
Lower*
Lower*
------
< 0.01†
< 0.01†
------
All Sites was analyzed with and without site 4 and p values were the same for both.
*, indicates significant difference between sites at α=0.05.
3
†, indicates difference was significant after Bonferroni correction.
2
P Value3
< 0.01†
50
Shannon diversity indices were significantly lower in control sites overall, but this
was not consistent for all sites. MPA site 1 had Shannon diversity value of 1.6 with a
shallow site value of 1.4 and a deep depth value of 1.8. Control site 1 had an overall
Shannon diversity value of 1.9 with a shallow depth value of 1.7 and a deep depth value
of 2.0. While site 1 overall yielded a significant difference between MPA and control
sites, only the shallow level of site 2 yielded significant differences when compared by
depth alone (shallow p<0.01; deep p=0.10.
Site 2 Shannon diversity indices showed significant differences between the MPA
and control sites overall (p<0.01) and in both shallow and deep depths (p<0.01 for both).
MPA site 2 had a Shannon diversity value of 1.6 with a shallow value of 1.5 and a deep
value of 1.6. Control site 2 had a Shannon diversity value of 2.2 with a shallow value of
2.1 and a deep value of 2.2. All levels showed a 25% higher value in the control sites
relative to the MPA sites for site 2.
MPA site 3 had a Shannon diversity value of 1.4 with a shallow value of 1.2 and a
deep value of 1.7. Control site 3 yielded Shannon diversity values of 1.6 (overall), 1.4
(shallow) and 1.9 (deep). None of these values were shown to be statistically different
(overall p=0.19; shallow p=0.28; deep p=0.26). Site 4 did show a statistically significant
difference between both the overall Shannon diversity values of the MPA and control
sites (p<0.01) as well as the shallow values (p<0.01). The overall and shallow value for
MPA 4 was 1.6. There was no deep value as there was no deep data set. The overall
value for Control 4 was 2.1 with shallow and deep Shannon diversity values also both at
2.1. These values are listed with their relative comparisons and p values in Table 7.
51
Table 7. Analysis of Shannon diversity index by site and overall sites.
Shannon
Shannon
MPA vs.
Diversity
Diversity
Control by
Index
Index
MPA relative
Site Number
MPA
Control
to Control2
All Sites1
1.52
1.94
Lower*
1
Site 1 Total
Shallow
Deep
1.55
1.40
1.78
1.88
1.73
2.05
Lower*
Lower*
Lower
< 0.01†
0.02†
0.10
Site 2 Total
Shallow
Deep
1.56
1.52
1.61
2.16
2.10
2.21
Lower*
Lower*
Lower*
< 0.01†
< 0.01†
< 0.01†
Site 3 Total
Shallow
Deep
1.44
1.16
1.72
1.60
1.37
1.86
Lower
Lower
Lower
0.20
0.28
0.27
Site 4 Total
Shallow
Deep
1.60
1.60
-----
2.10
2.10
2.10
Lower*
Lower*
-----
< 0.01†
< 0.01†
------
All Sites was analyzed with and without site 4 and p values were the same for both.
*, indicates significant difference between sites at α=0.05.
3
†, indicates difference was significant after Bonferroni correction.
2
P Value3
< 0.01†
52
The interaction between depth and site location was also analyzed with respect to
the three biodiversity indices on a site by site basis. Graphical analysis showed the
primary site of interaction for control sites was site 3 for percent cover, whereas MPA
managed sites showed site depth-location interactions at site 1 for percent cover and site
2 for both species richness and Shannon diversity. These graphical depictions are
illustrated in Figure 7.
53
A
B
C
Figure 7. Interactions and variation when separated by depth and site number.
Bonferroni corrected ANOVAs of percent coral cover (A), species richness (B)
and Shannon diversity (C) on a site by site basis. These values represent both
depth ranges combined. Error bars represent 95% confidence intervals.
54
DISCUSSION
Qualitative Site Observations
My initial observations on sea turtles and fishes support the idea that MPAs are
not acting as the sources of biodiversity that they are intended to be. As Howard et al.
inferred in his 2009 study, the presence and diversity of parrotfish as well as other
herbivorous fish are indicator species within a coral reef environment. When these fishes
are removed, a reduction of benthic epifauna and coral cover is observed (Howard et al.,
2009). These observations were supported in my study as well; I saw significantly fewer
numbers of parrotfishes at MPA sites relative to control sites.
Regarding juvenile fishes, both myself and my research divers observed higher
numbers and more active behaviors of juvenile fishes in the control regions than those of
the MPAs. There are many possible explanations for these observations, but if MPAs are
acting as protected areas for fishes, one might expect to see juvenile fishes lingering in
those areas until a more defensible size or more adept defensive skills are achieved.
Furthermore, in areas with no protective status, one might expect to see more skittish or
defensive behaviors due to higher risk; however, the juvenile fishes were openly
swimming and feeding, even with divers in the area. The cause of this behavior, again, is
unclear and cannot be explained within the constraints of this study. It may be due to
effects of management status or the larger numbers of juvenile fishes may be the result of
some other factor (e.g. fish feeding in control areas). More observations and larger data
sets are required to make any concrete conclusions regarding this observation.
55
Unlike juvenile fishes, sea turtles were observed in moderate numbers at all sites,
but in larger numbers at two specific sites: Mokuleia (MPA site 4) and Turtle Town
(control site 1). While is clear that turtles often occur in large numbers at the site referred
to as Turtle Town, there is less evidence or public knowledge of turtle concentrations in
Mokuleia and less of an explanation for this congregation. Turtle Town hosts an
abundant turtle population because the area offers lava tubes, arches and crevices for
turtles to hide in (Hoover, 1993), while supplying prolific amounts of algae due to its
proximity to point source pollution sites (Hoover, 1993; Sazima et al., 2010). Mokuleia
has no observable point source pollution site, nor does it offer any sort of visible
protective refuge. Is the presence of turtles in MPA 4 an indication of that MPA’s
success (turtle protection) or failure (turtle access to encroaching algae)? Future studies
may answer this question and shed light on the larger picture of Maui County’s MPAs.
In this, I suggest a study of nekton, sea turtles or other macrofauna be attempted in order
to see the bigger picture.
Tourism Observations
The observed differences in tourist counts at each site are consistent with the
islands tourism demographics: the north shores of Maui (site 3) contain more time shares
and more leisure based activities, while the south shores of Maui (sites 1 and 2) cater to
families, activity-abundant resorts, and a higher activity level (Hawaii.gov, 2010).
Weather on the south shores of Maui is also more conducive to year-round water activity
because the south shores of Maui are on the leeward side of the island and therefore,
56
more protected from wave-inducing trade-winds. This disparity in activity types is also
likely due to differences in island shelf slope (Sinton, 2003) because a shallow beach
slope and clear waters (south shores) make tourist access easier and safer. Site 4 is on the
leeward side of the island of Lana’ i, which experiences lower rates of tourism because of
its exclusivity. Despite the differences in demographic-based tourism dispersal, the
MPAs experienced a higher visitor turnout relative to their paired control sites.
Overall Analysis: Marine Protected Areas compared to control group
The significance of the main effect observed indicates that MPAs are showing a
lower biodiversity relative to biogeographically similar controls. Given that the goal of
these MPAs is to increase biodiversity by acting as a refuge and eventual source of larval
recruitment, these results are contrary to what is intended. One would hope to see
ecosystem development near that of a climax community in these MPAs, so that
migration of large numbers of both adult and larval forms of species would take place,
compensating for over-fished, over-exploited reefs nearby. Instead this study suggests
the MPAs are not living up to their intended goal and observations of degraded coral and
limited fish diversity support this.
Because the MPA biodiversity indices were significantly lower than their relative
controls, rather than higher or not significantly different, I feel this study clearly implies a
problem with the Marine Protected Areas currently in place in Maui County. The marine
ecosystem is both dynamic and fluid in character; therefore, an insignificant variance in
biodiversity would not have indicated failure or success of the MPA system as clearly as
57
the observed results do. For example, an MPA with biodiversity indices similar to its
control site may exhibit this similar biodiversity because it effectively sourced sink
populations or non-conserved areas and thereby balanced the two groups, causing an
unnoted increase in the control sites (Syms and Carr, 2009). Thus, the balanced state, or
equal biodiversity indices of the two sites might indicate a successfully regulated MPA,
but this can really only be supported through studies of the control sites over time, as
Stevenson and Tissot did in their 2009 study off the Northwest Kona Coast. Conversely,
a seemingly balanced set of biodiversity indices could indicate failure of the MPA on
many levels. It may indicate normal use due to lack of enforcement or heightened use
and therefore increased degradation of conservation areas based on appeal and tourism
draw. It may also indicate poor placement of MPA boundaries due to degradation factors
in close proximity to that MPA, or failure to designate an MPA in time to salvage an
already degraded area. In short, in such an open and dynamic system, balancing and
equalizing processes are the norm and can be the result of many different factors;
therefore insignificant differences in biodiversity indices alone can tell an observer very
little about the system he or she is observing.
Significantly lower biodiversity indices within MPAs, like those seen in the
results of this study, are clear indications of a problem with some aspect of those areas.
Whether the problem is management based, or rooted in other potential problems like
those listed above, is unclear from these results. In the case of this study, the overall
differences between MPA and control sites were both drastic and significant when
viewed as a whole. When viewed as smaller subsets, however, the drastic disparities
58
between the MPAs and control sites seem less significant and less consistent across sites.
When one considers the unique idiosyncrasies of each site, the differences across sites
with respect to depths and interactions are more understandable, and the overall main
effect is substantiated: tourism is adversely affecting coral reef diversity and resilience.
Site 1 Analysis
Mean biodiversity indices were significantly higher in control sites relative to
MPA- managed sites. The one exception of to this trend was in the category of percent
cover in which the MPA-managed site exhibited significantly higher mean percentages.
A likely explanation for this exception is the sensitivity of coral paired with the higher
rates of usage in the control sites of site 1. The tourist count data supports this, reporting
four times as many tourists at the site 1 control sites. Although, tourist counts were not
obtained previous to this study, recent events in south Maui may be the cause of these
lower tourist numbers in MPA-managed site 1.
In an 8-1 committee vote, an advisory panel for the Department of Land and
Natural Resources (HDLNR) voted (November 16, 2004) to close these terrestrial foot
paths and associated marine areas (referred to as the “fishbowl”) within the Ahihi-Kinau
reserve due to excessive recreational use and resultant degradation. At the time of data
acquisition for this study, these areas had been closed to the public for nearly two years;
consequently, none of the areas already determined to be degraded were used (HDLNR,
2010). Instead, areas within the preserve but surrounding the closed area were used in an
attempt to reduce biased results. However, the closure of this highly used area may have
59
deterred tourists from visiting the reserve altogether and contributed to the low tourist
rates relative to other MPA sites.
The three control sites sampled to obtain the sample set are all, however, in the
same vicinity of MPA site 1: South Maui. This region is well known as a robust tourism
area with many resorts, vacation rentals, condos and private residences. The region is
highly used by both tourists and residents and is the most easily accessible to tourists
staying in South Maui. MPA site 1 is also highly used but potentially less so now that the
“fish bowl” has been closed to the public.
Given the fragile nature of coral, percent cover is likely to be the first index to
show degraded states in areas of over-use (Mumby and Hastings, 2008). The observation
that only percent cover is significantly lower in control site 1 may indicate that the other
biodiversity indices in this control site may soon decrease. The indices of species
richness and Shannon diversity both rely on the resilience and abundance of the coral
substrate within a coral reef community. The lower percent cover of control site 1 may
be due to the increased usage of that site after the closure of sections of MPA site 1. This
observation may also serve as a harbinger of reduced species richness or Shannon
diversity indices.
The lack of significance in the comparison of the two management types at site 1
is most likely due to recent changes in this region (mentioned above) as well as the
variation of results at the individual depth levels.
60
Site 2 Analysis
MPA site 2 exhibited lower mean biodiversity indices and higher usage relative to
its control. This again supports the hypothesis that MPAs are experiencing higher usage
and therefore subject to higher levels of degradation.
In 2009, the recreational diving company, Maui Dive Company. was fined
$650,000 for destruction of a portion of reef within the reserve when one of their boats
that used the site daily, sunk on the east side of the islets interior (Maui News, 2010).
Both the MPA and the control for site 2 experience larger than typical amounts of boat
use. However, boats use MPA 2 almost constantly, the results of this study indicate
significantly lower biodiversity indices in MPA 2 relative to its control. Therefore, it is
possible that much of the degradation, but not all, is due to boat pollution or anchor
damage, rather than snorkeler or diver damage. This supports the findings of Jameson
(1999), who found marine areas with higher rates of boat usage to have higher levels of
degradation. Future studies might consider deciphering how much damage is caused by
each at a site like this.
In the analysis of site 2, it is important to consider that unlike other sites, site 2
control and site 2 MPA both have active lifeguarding. Many of the boats using the areas
sampled have lifeguards who are not only trained in safety protocol but also in
conservation protocol. These lifeguards know that snorkelers and divers should not touch
coral or other marine life, and these lifeguards are trained to prohibit tourists from doing
so (Pacific Whale Foundation, 2006). Despite the precautions taken by ecotourism
companies, the results of this study indicate that MPAs have lower biodiversity indices
61
than their biogeographically similar controls. This suggests that although attempts are
being made to mitigate the degradation caused by tourists, in areas with high tourist draw,
such as MPA 2, the mitigation is not enough.
Site 3 Analysis
Site 3 showed no significant difference in Shannon diversity indices. The major
contributing factor in this lack of significance was the variance of the samples from each
management area. Mala Dock, as the name implies, was at one time a public dock, which
collapsed during 1992’s Hurricane Iniki. The coral ecosystem that now exists is newly
established coral anchored on acceptable substrate and therefore is an example of a new
reef, forming or recovering on its own accord. This control site is similar to its MPA site,
Honolua, in that Honolua too has experienced structural damage in the form of landslides
due to heavy rain from the adjacent West Maui Mountains (Honolua, 2009; Maui News,
2010). The difference between the two is observable; there is little coral life in the
landslide affected areas while the collapsed dock area has substantial new growth. The
variance in these sites occurs for two reasons: areas of high diversity and decent percent
cover paired with damaged areas of no diversity and no percent cover. Of the three
transects for Honolua, one was found within the area affected by landslides while all
three transects were within the Hurricane-affected areas of Mala docks.
Furthermore, it is important to note, that while diversity was not always
significantly lower in the MPA site 3, it was always close to being significantly lower and
would have been considered significantly lower in all cases without the Bonferroni
62
correction, despite the fact that the control site had experienced more overall damage per
data point. However, because it is highly likely that the Mala docks site is not a true
representative sample of the possible control areas for MPA site 3, it is my opinion that a
larger data set with samples from other northwest shore sites would better substantiate the
results found in this study. In light of this evidence, it is all the more telling and
impressive that percent coral cover was still significantly higher in the control site 3
relative to MPA site 3.
The diversity indices of MPA site 3 (Honolua) also sharply contradict previous
studies on this site. In his 1994 study of fish populations in protected and unprotected
areas in Hawaii, Grigg found Honolua to have significantly higher numbers of fish and
percent cover than its control site (Grigg, 1994). This was not the case for observations
made in the current study; percent cover in MPA 3 was lower than its control and the
dive team observed fewer fish as well. The other Maui County MPA site in his study,
Molokini, showed no significant difference in fish population biodiversity but a
significantly lower percent cover relative to its control site, which is more consistent with
the findings of this study (Grigg, 1994). Although the benthic focus of my study differs
from that of Grigg, the interconnectedness of the benthic epifauna and fishes of these
coral reefs insinuates that over the past twenty years we have seen a decline in coral reef
biodiversity in Honolua Bay and in Molokini Crater. It is upsetting to think that
protected areas did not show increases in biodiversity over time as is seen in the case of
Molokini; it is devastating to witness evidence of a decrease in biodiversity as in the case
of Honolua.
63
Site 4 Analysis
Site 4 was only analyzed in the shallow-samples-pair as a deep sample-set for site
4 did not exist. Hulopo'e Bay is a no boat access zone with patch reef on the sides of the
bay but large sandy underwater plains in the central, deeper portions. Hulopo'e’s
counterpart, Manele Bay does possess deep coral reef habitats; however, these areas exist
on either side of a high traffic boat channel, so there is some risk in accessing it. Despite
this danger, many tourists still use the area for snorkeling, but to less effect than the
easily accessed Hulopo’e Bay. Throughout the available sampling time, it was
determined that accessing Manele Bay was too dangerous due to weather or boat traffic
conditions. Also during this time, there was no evidence of tourist use, likely for the
same reasons. Like Lana’ i, control site 4, Olowalu, has boat and weather hazards on
occasion. Olowalu is a popular destination for boat-based snorkeling so boat traffic is
always considered. Olowalu is also a popular shore access snorkeling site, but in order to
reach the depths within the sampling range for this study, it is necessary to swim
distances beyond the typical shore-snorkeler’s range. Therefore, the sampling area for
this site experiences mostly boat-based snorkeling and diving but also the occasional inshape snorkeler or diver.
Despite these similarities, the lack of a deep MPA site 4 data set was recognized
as a possible source of error and potential violation of statistical (ANOVA-based) rules,
and so all Site 4 inclusive analyses were performed twice: once including all data from
sites 1-4 and once excluding all data from site 4 (sites 1-3 only). Analysis of the reduced
data set still indicated that the differences between the MPA and control sites were
64
significantly different across all tested biodiversity indices (percent cover, species
richness, Shannon diversity. Careful attention to potential sources of error and clear
elimination of these sources of doubt regarding the significance of these differences,
further bolsters my claim that there is a clear and significant difference between the
biodiversity indices PC, S and H’ in MPAs and similar control sites.
65
Table 8. Mean descriptive statistics of all Marine Protected Areas and control sites per
site number.
Site Management
Type and Number1
Mean Number of
Tourists 2
Mean
Percent
Cover3
Mean
Species
Richness4
Mean
Shannon
Diversity5
MPA 1
24
34%
8.0*
1.55*
Control 1
100
28%
9.3*
1.88*
MPA 2
237
36%*
7.2*
1.56*
Control 2
3
61%*
12*
2.16*
MPA 3
86
4.2%*
6.2*
1.44
Control 3
45
22%*
7.4*
1.60
MPA 4
123
22%*
8.9*
1.60*
Control 4
50
63%*
12*
2.10*
1
includes both shallow and deep depth ranges
based on mean per time at each site
3,4,5
*, indicates significant differences at α= 0.05
2
66
Depth Analysis
Based on observed results, we can conclude that depth does play a role in the
biodiversity of a coral reef in the waters of Maui County. Both MPAs and controls
showed a change in biodiversity with increased depth. While there was no consistent
trend in percent cover differences across the depth ranges, species richness and Shannon
diversity indices were significantly lower in the shallow depths. This supports the
findings of Hasler and Ott (2008), who found that diver-based damage decreased with
movement away from shore. Hasler and Ott’s explanation for this was based on diver
entry sites. However, if damage observed is in fact due to diver-caused trauma, one
would expect to see a decrease in coral cover in shallow regions as well; this is the area
where many divers and snorkelers can be observed stepping or walking on coral. It is
also the area where divers experience the most pressure change and thus, have the most
problems with buoyancy control.
Some sites showed lower percent coral cover at deep depths relative to their
shallow counterparts. These sites (control 1, MPA 2, control 2, and control 4) all contain
mooring anchors throughout the site within the deep depth range. Commercial recreation
boats use these moorings daily to tie up their boats while divers and snorkelers use the
area. Consequently, areas around these moorings experience higher tourist use and
potentially may experience more pollution and physical damage from boats. The
findings of this depth analysis support this idea because the areas that harbor these
moorings show decreased damage at the mooring depth, whereas the “deep” areas
67
without moorings in or near the deep depth range (MPA 1, MPA 3, control 3, MPA 4) did
not exhibit this lower biodiversity relative to their shallow counterparts.
Future Studies
Other observations worth mentioning but that cannot be inferred from the given
statistical analysis may serve as topics of a secondary study or a supplementary study to
this one. The first observation is with respect to the distinct differences of samples
yielding zero for the three indices tested. These “zero” samples show up statistically as
the same thing, but upon closer observation might indicate very different events, states or
qualities of the ecosystem in question. For example, many of the zero samples within
control sites are the result of sand channels between healthy coral (82%) or sand around
patch reef, which is a normal aspect of reef ecosystems. Many of the MPA site zero
samples were littered with dead coral or algae (70%), a clear indication of a now
degraded area that was recently a functioning reef, the primary biological focus of this
study.
Another important consideration in the implications of this study and future
follow-up studies is based on the long-debated, still elusive concept of species. The
biological species concept, one focused on defining species as capable of interbreeding,
has yet to reconcile the placement of many invertebrate animals and plants, and most
bacteria and fungi. Not only has this been problematic in policies such as the ESA (Bean,
2009), but it creates source of artifact or inaccuracy when trying to account for
biodiversity and species richness.
68
Another source of uncertainty is in the counting protocol of clonal, aggregate or
endosymbiont species such as sponges, tunicates, corals and parasites. Given that these
animals rarely exist alone, many would like to refer to the unit as one individual. Often,
this leaves the researcher with one individual per sampling area, instead of thousands.
Clearly, a change of this magnitude can affect biodiversity indices. In the case of
endosymbiotic or parasitic species, it is sometimes unclear how many organisms are
living within one organism and whether or not they should be counted separately. While
this artifact can be mitigated through consistency in approach, it is possible that a study
which includes microscopic organisms, while potentially more invasive, may yield
different or more accurate species counts.
Based on this second observation, is a third that merits additional study: that of
scale of system. I argue that the scale of organisms, interactions and microenvironments
is small enough in the coral reef system to consider samples independent in the predetermined sample areas. It is possible, based on this concept that a more microscopiclevel study may be valuable in substantiating or refuting the above study. Not only
would a smaller-scale study yield more organisms at the microscopic level, but it would
allow a cellular-organism analysis of those animals that may be considered individuals at
the cellular level.
69
CONCLUSION
The results of this study are clear and potentially significant. The touristaccessible MPAs of Maui County experience higher rates of usage and display lower
biodiversity indices than their biogeographically similar control groups. The current
MPAs in place in Maui County are in need of review and revision if these areas are at
risk of greater degradation because of their designation as protected areas. Effective
implementation of Marine Protected Areas should involve both scientific opinion and the
opinions of community stakeholders (Fearon, 2003), because MPA regulations need to be
sustainable in order to be effective. It is necessary, however, to balance the needs of all
stakeholders involved. In the case of these MPAs, it seems clear that in order to preserve
the quality of human life in Maui County, many biological aspects of conservation have
been overlooked. It is possible that tourist-attraction to these sites had not been
considered at the time of MPA establishment. Factors involving tourism have only
recently been considered with respect to MPAs (Meyer and Holland, 2009).
Modifications that allow sustainable ecotourism methods have been suggested and
reviewed by Hawkins et al. (2005) and Walters and Samways (2001). I suggest that Maui
County’s policy makers consider not only implementing these ideas, but adding one new,
highly-restricted MPA for every tourist-accessible MPA in Maui County.
It would be economically devastating to restrict usage of the current MPAs;
tourism is the County’s primary source of revenue and without the draw of these areas,
tourism would suffer. In light of the results of my study, however, one can foresee an
economic downfall for Maui County if nothing is done to mitigate the excessive use of
70
these areas. This is already evident in the closure of portions of one of Maui’s MPAs.
Perhaps new, highly-restricted MPAs will act as true source populations, while the
existing MPAs will continue to draw tourists without hurting the goals of conservation.
In his 1921 discussion of Ocean Pasturage, W.E. Allen said “We can obtain little
information about the global conditions through a single droplet of water”, but what we
can be sure of is that even that single droplet of water is capable of acting as the impetus
of change. Likewise, individual humans, while more capable of causing obvious and
significant events when in mass, can change the direction of events with their policies,
paradigms and personal decisions. A focus on ecology with the human component is
essential to the success of any policy to be respected and observed by humans. When we
succeed in this marriage of ecology and humanity, required rule enforcement will be “a
rare event”, as Kirlin stated in his 1993 analysis. However, true success in conservation
may require not only a more holistic view of the needs of all stakeholders, but also a
paradigm shift with respect to humankind’s true place in the system.
71
LITERATURE CITED
Agardy, T. (1994) Advances in marine conservation: The role of marine protected areas.
Trends in Ecology and Evolution 9: 267-270.
Almany, G.R., S.R. Connolly, D.D. Heath, J.D. Hogan, G.P. Jones, L.J. McCook, M.
Mills, R.L. Pressey and D.H. Williamson (2009) Review: Connectivity,
biodiversity conservation and the design of marine reserve networks for coral
reefs. Coral Reefs: online: 10.1007/s00338-009-0484-x
Avery, W.E. (1998) Bahamian Reef Communities: Composition, Recruitment and
Change (10 meters to 250 meters). Ph.D. Dissertation: Utah State Univerity.
Ban, N., C. Picard and A. Vincent (2009) Comparing and integrating community-based
and science-based approaches to prioritizing marine areas for protection.
Conservation Biology 23: 899-910.
Barker, N.L., C.M. Roberts (2004) Scuba diver behaviour and the management of diving
impacts on coral reefs. Biological Conservation 120: 481-489.
Bean, M.J. (2009) The Endangered Species Act: Science, Policy and Politics. New York
Academy of Sciences 1162: 369-391.
Bellwood, D.R. and J.H. Choat (1990) A functional analysis of grazing in Parrotfishes
(family Scaridae): the ecological implications. Environmental Biology of Fishes
28: 189-214.
Brock, R.E. (1979) An Experimental Study on the Effects of Grazing by Parrotfishes and
Role of Refuges in Benthic Community Structure. Marine Biology 51: 381-388.
72
Burgess, S.C. (2006) Algal blooms on coral reefs with low anthropogenic impact in the
Great Barrier Reef. Coral Reefs 25: 390-391.
Byrne, J.E. (1970) Mucous Envelope formation in 2 species of Hawaiian Parrotfishes
(Genus Scarus). Pacific Science 24: 490-493.
Cardwell, J.R., N.R. Liley (1991) Hormonal control of sex and color change in the
stoplight parrotfish, Sparisoma viride. General and Comparative Endocrinology
81: 7-20.
Danovaro, R., L. Bongiomi, C. Corinaldes, D. Giovannelli, E. Damiani, P. Astolfi, L.
Greci, A. Pusceddu (2008) Sunscreens cause coral bleaching by promoting viral
infections. Environmental Health Perspectives 116: 441-447.
Fearon, R. (2003) Linking stakeholders and decision makers with science in managing
the coastal water environment: case studies from urban, industrial and rural
subtropical catchments in Australia. Water Science and Technology 47: 179-184.
Fedorowski, J. (2009) Morphogenesis and taxonomic value of the circumaxial skeleton in
Rugosa (Anthozoa). Lethaia 42: 232-247.
Ferreira, C.E., J. L. Gasparini, A. Carvalho-Filho, S. R. Floeter (2005) A recently
extinct parrotfish species from Brazil. Coral Reefs 24: 128.
Garcia-Sais, J.R. (2010) Reef habitats and associated sessile-benthic and fish
assemblages across a euphotic-mesophotic depth gradient in Isla Desecho, Puerto
Rico. Coral Reefs online: DOI 10.1007/s00338-009-0582-9.
73
Gardner, T. A., I. M. Cote´, J.A. Gill, A. Grant and A. R.Watkinson (2005) Hurricanes
and Caribbean coral reefs: impacts, recovery and role in long-term decline.
Ecology, 86: 174–184.
Grigg, R.W. (1994) Effects of sewage discharge, fishing pressure and habitat complexity
on coral ecosystems and reef fishes in Hawaii. Marine Ecology Progress Series
103: 25-34.
Guyton, A.C. and J.E. Hall (2005) Textbook of Medical Physiology 10th ed. online
edition. WB Saunder Company: US.
Harding, E.K., E.E. Crone, B.D. Eldred, J.M. Hoekstra, A.J. McKerrow, J.D. Perrine, J.
Regetz, L.J. Rissler, A.G. Stanley, E.L. Walters, and NCEAS Habitat
Conservation Plan Working Group (2001) The Scientific Foundations of Habitat
Conservation Plans: a Quantitative Assessment. Conservation 15: 488-500.
Hassler, H. and J.A. Ott (2008) Diving down the reefs? Intensive diving tourism threatens
the reefs of the Northern Red Sea. Marine Pollution Bulletin 56: 1788-1794.
Hawai’i Department of Commerce (2010) http://hawaii.gov
Hawai’i Department of Land and Natural Resources, Aquatics division (HDLNR) (2010)
http://hawaii.gov/dlnr/dar/
Hawkins, J.P., C.M. Roberts, D. Kooistra, K. Buchan and S. White (2005) Sustainability
of SCUBA diving tourism on coral reefs of Saba. Coastal Management 33: 373387.
Hixon, M.A. and W. N. Brostoff (1985) Substrate characteristics, fish grazing, and
epibenthic reef assemblages off Hawaii. Bulletin of Marine Science 37: 200-213.
74
Hoover, J.P. (1998) Hawaii’s Sea Creatures: A guide to Hawaii’s Marine Invertebrates.
Honolulu: Mutual Publishing.
Hoover, J.P. (1993) Hawaii’s Fishes: A guide for snorkelers, divers and aquarists.
Honolulu: Mutual Publishing.
Howard, K.G., B.D.Schumacher and J.D.Parrish (2009) Community structure and habitat
associations of Parrotfishes on Oahu, Hawaii. Environmental Biology of Fishes.
Hubbell, S.P. (1997) A unified theory of biogeography and relative species abundance
and its application to tropical rain forests and coral reefs. Coral Reefs 16: S9-S21.
International Union for Conservation of Nature (IUCN) (2010) http://www.iucn.org/
Jameson, S.C., M.S.A. Ammar, E. Saadalla, H.M. Mostafa and B. Riegl (1999) A coral
damage index and its application to diving sites in the Egyptian Red Sea. Coral
Reefs 18: 333-339.
Kahoolawe Island Reserve Commission (KIRC) (2009) http://kahoolawe.hawaii.gov/
Karleskint, G. (1998) Introduction to Marine Biology. Chapter 6. Orlando: Harcourt
Brace College Publishing.
Kirlin, J.J., P. Asmus and R. Thompson (1993) Conservation and Cooperation: Strategies
for making endangered species laws work. California department of Fish and
Game: June 1993.
Klugh, B. (1924) Factors controlling the biota of tide-pools. Ecology 5: 192-196.
Krebs, C.J. (1999) Ecological Methodology 2nd ed., pages 64-65. New York: Harper and
Row.
75
Kuwahara, V.S., R. Nakajima, B.H.R. Othman, M.R.M. Kushairi and T. Toda (2010)
Spatial variability of UVR attenuation and bio-optical factors in shallow coralreef waters of Malaysia. Coral Reefs online: DOI 10.1007/s00338-010-0618-1.
Lehman, S., G. Kaufman, K.M. Thomasson and J. Devore, (2006) Humpback whale and
commercial boat interaction: Curiosity or Avoidance? Pacific Whale Foundation
Archives: unpublished manuscript.
Littler, M.M., P.R. Taylor and D.S. Littler (1983) Algal Resistance to Herbivory on a
Caribbean Barrier Reef Coral Reefs 2: 1l1-118.
MacArthur, R.R. and E.O. Wilson (1967) The theory of island biogeography. Princeton,
New Jersey: Princeton University Press.
Manzello, DP. (2010) Coral growth with thermal stress and ocean acidification: lessons
from the eastern tropical pacific. Coral Reefs: online: DOI: 10.1007/s00338-0100623-4.
Marine Protected Area Commission (MPAC) (2009) http://mpa.gov
Maui News (2010) Kahului, Maui. Mauinews.com/archives/
McClanahan T.R. and S.H. Shafir (1990) Causes and consequences of Sea Urchin
abundance and diversity in Kenyan coral reef lagoons. Oceologia 83: 362-370.
McKindsey, C. and E. Bourget (2001) Diversity of a Northern Rocky intertidal
community: The influence of body size and succession. Ecology 82: 3462-3478.
Meyer, C.G. and K.N. Holland (2009) Spatial dynamics and substrate impacts of
recreational snorkelers and SCUBA divers in Hawaiian Marine Protected Areas.
Journal of Coastal Conservation 12: 209-216.
76
Molodtsova, T. , N. Sanamyan and N. Keller (2008). Anthozoa from the northern midAtlantic ridge and charlie-gibbs fracture zone. Marine Biology Research 4: 112130.
Mora, C., A. Andre` fouet, M.J. Costello, C. Kranenburg, A. Rollo, J. Vernon, K.J.
Gaston, R.A. Myers (2006) Coral reefs and the global network of Marine
Protected Areas. Science 312: 1750-1751.
Mumby, P.J. (2006) The impact of exploiting grazers (Scaridae) on the dynamics of
Caribbean coral reefs. Ecological Applications 16: 747-749.
Mumby, P.J. and A. Hastings (2008) The impact of ecosystem connectivity on coral reef
resilience. Journal of Applied Ecology 45: 854-862.
Mumby, P.J. and A. Harborne (2010) Marine Reserves enhance the recovery of corals on
Caribbean Reefs. PLos One: online: DOI: 10.1371/journal.pone.0008657.
Munday, P.L., J.M. Leis, J.M. Lough, C.B. Paris, M.J. Kingsford, M.L. Berumen, J.
Lambrechts (2009) Climate change and coral reef connectivity. Coral Reefs 28:
353-366.
Nation Oceanic and Atmospheric Administration (NOAA) (2010) www.noaa.gov
O’Conner, M.I., J.F. Bruno, S.D. Gaines, B.S. Halpern, S.E. Lester, B.P. Kinlan, J.M.
Weiss (2007) Temperature control of larval dispersal and the implications for
marine ecology, evolution and conservation. Proceedings of the National
Academy of Sciences 104: 1266-1271.
Pacific Whale Foundation (2006) Lecture Series online. www.pacificwhale.org
77
Riegl B, A Bruckner, SL Coles, P Renaud and RE Dodge (2009) Coral Reefs: Threats
and Conservation in an Era of Global Change. New York Academy of Sciences
1162: 136-186.
Planes, S., G.P. Jones and S.R. Thorrold (2009) Management under uncertainty: guidelines for incorporating connectivity into the protection of coral reefs. PNAS 106:
5693-5697.
Save Honolua Coalition (2009) savehonolua.org
Sazima, C., A. Grossman and I. Sazima (2010)Turtle cleaners: reef fishes foraging on
epibionts of sea turtles in the tropical Southwestern Atlantic, with a summary of
this association type. Neotropical Ichthyology 8: 187-192.
Schutter, M., J. Crocker, A. Paijmans, M. Janse, R. Osinga, A.J. Verreth, R.H. Wijffels
(2009) The effect of different flow regimes on the growth and metabolic rates of
scleractinian coral Galaxia fascicularis. Coral Reefs online: DOI 10.1007/s00338010-0617-2.
Sinton, J.M. (2003) Geologic History of Maui. Hawaii Institute of Geophysics: Chapter
1. University of Hawaii Press, Honolulu.
Smith, T.B., P. Fong, R. Kennison, and J. Smith (2010) Spatial refuges and associated
defenses promote harmful blooms of the alga Caulerpa sertularioides onto coral
reefs. Oecologia DOI 10.1007/s00442-010-1698-x
Stevenson T.C. and B. Tissot (2009) Hawaiian Islands ecosystem case study: Ecosystemand community-based management in Hawaii. Coastal Management 37: 255-273.
78
Streelman, J.T., Alfaro, M. W. Westneat, D. R. Bellwood and S. A. Karl (2002)
Evolutionary History of the Parrotfishes: Biogeography, Ecomorphology, and
Comparative Diversity Source: Evolution 56: 961-971.
Syms, C. and M.H. Carr (2009) Marine Protected Areas: Evaluating MPA effectiveness
in an uncertain world. UCSC lecture series.
Tratalos, J.A. and T.J. Austin (2001) Impacts of recreational SCUBA diving on coral
communities of the Caribbean island of Grand Cayman. Biological
Conservation 102:67-75.
Tewfik, A., J.B. Ramussen and K.S. McCann (2005) Anthropogenic enrichment alters a
marine benthic food web. Ecology 86: 2726-2736.
Van Treeck, P. and H. Schumacher (1998) Mass diving tourism- A new dimension calls
for new management approaches. Marine Pollution Bulletin 37:499-504.
Videlier, H., G.J. Geertjes, and J.J. Videlier (1999) Biochemical characteristics and
antibiotic properties of the mucous envelope of the queen parrotfish. Journal of
Fish Biology 54: 1124–1127.
Walters, R.D.M. and M.J. Samways (2001) Sustainable dive ecotourism on a South
African coral reef. Biodiversity and Conservation 10:2167-2179.
Westneat, M. W. and M. E. Alfaro (2005) Phylogenetic relationships and evolutionary
history of the reef fish family Labridae. Molecular Phylogenetics and Evolution
36: 201-428.
Weis, V.(2010) The susceptibility and resilience of corals to thermal stress: Adaptation,
acclimatization or both?. Molecular Ecology 19: 1515-1517.
79
Zelnio, K. , E. Rodriguez and M. Daly. (2009) Hexacorals (anthozoa: Actiniaria,
zoanthidea) from hydrothermal vents in the south-western pacific. Marine
Biology Research 5: 547-571.