Organic Chemistry Outline
Organic Chemistry
Outline
Organicoutline06.doc
The study of the compounds of carbon (exceptions: CO,
CO
2
, CO
3
2, etc.)
Carbon has a unique ability to form long chains with fairly stable bonds.
Since carbon has a tetrahedral geometry there will always be 4 bonds to each carbon atom
C
For simple carbon compounds known as hydrocarbons, hydrogen is found to fill in (saturate) non carbon-carbon bonds
Ex.
H H H H
H C C C C H
H H H H
These types of compounds (those with only single bonds and containing only carbon and hydrogen) are known as alkanes or saturated hydrocarbons .
Alkane Nomenclature
The name of an alkane is dependant upon the number of carbons in the chain using the following prefixes:
3
4
5
6
# carbons
1
2
Prefix meth eth prop but pent hex
7
8
9
10 hept oct non dec
The suffix "ane" is added to designate the molecule as an alkane.
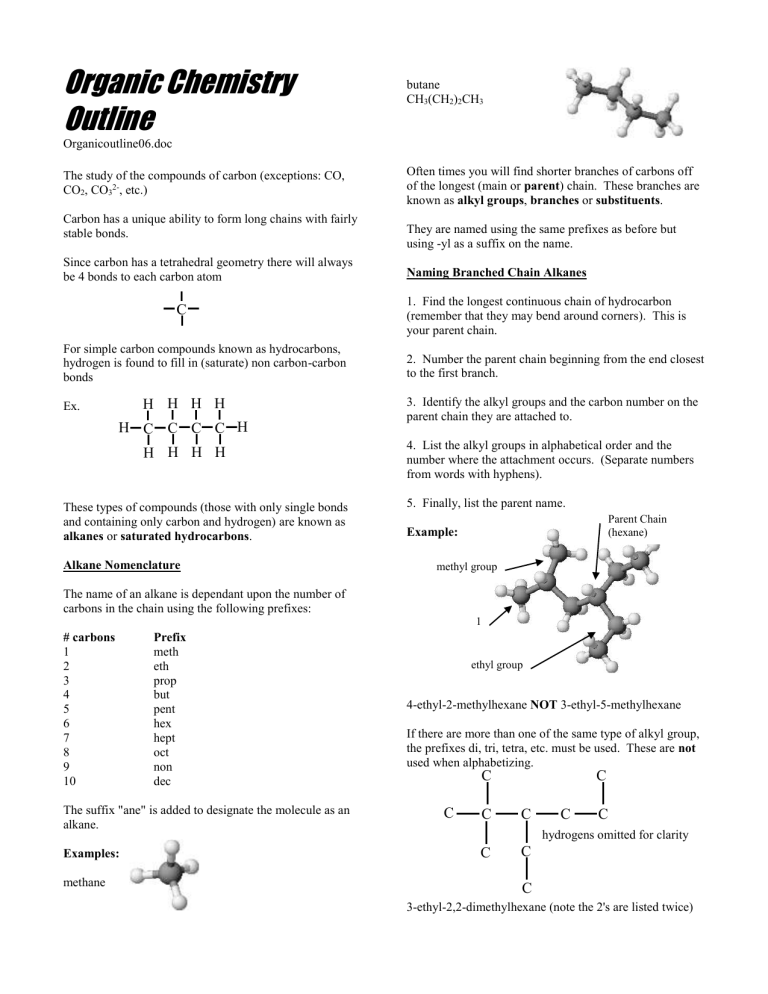
Examples: methane butane
CH
3
(CH
2
)
2
CH
3
Often times you will find shorter branches of carbons off of the longest (main or parent ) chain. These branches are known as alkyl groups , branches or substituents .
They are named using the same prefixes as before but using -yl as a suffix on the name.
Naming Branched Chain Alkanes
1. Find the longest continuous chain of hydrocarbon
(remember that they may bend around corners). This is your parent chain.
2. Number the parent chain beginning from the end closest to the first branch.
3. Identify the alkyl groups and the carbon number on the parent chain they are attached to.
4. List the alkyl groups in alphabetical order and the number where the attachment occurs. (Separate numbers from words with hyphens).
5. Finally, list the parent name.
Example: methyl group
Parent Chain
(hexane)
1 ethyl group
4-ethyl-2-methylhexane NOT 3-ethyl-5-methylhexane
If there are more than one of the same type of alkyl group, the prefixes di, tri, tetra, etc. must be used. These are not used when alphabetizing.
C C
C C C C C hydrogens omitted for clarity
C C
C
3-ethyl-2,2-dimethylhexane (note the 2's are listed twice)
Sidenote: Nonstandard branches
C - C - C Isopropyl
|
C - C - C - C
| sec-butyl
C
|
C - C
|
C
|
C
Isobutyl
|
C - C - C
| tert-butyl (tertiary)
The line on each substituent indicates where it attaches to the parent chain. Tert and sec are not used in alphabetizing but iso is because it is part of the name.
Sometimes organic compounds have the same chemical formula but different structures. They are known as structural isomers .
Ex. Each of the following molecules has the formula
C
4
H
10
C - C - C - C n-butane (where n stands for "normal" or straight chain)
C
|
C - C - C 2-methylpropane (or just methylpropane)
Therefore they are isomers of one another.
Compounds that contain one of more double bonds (two pair of electrons shared between carbons) are classified as alkenes and have the suffix ene in their name.
Carbon compounds containing one or more triple bonds are called alkynes and have the suffix yne . Double and triple bonds are no longer free to rotate like alkanes.
Naming these compounds is similar to alkanes but with a few very important exceptions.
1. The parent chain is the longest continuous chain of carbons containing all the multiple bonds.
2. Begin numbering the parent chain from the end closest to the first multiple bond (if it is the same then go to the next double or triple bond)
3. The name must indicate the quantity and location of the multiple bond(s).
4. If the carbon chains come off of the same side of the double bond it’s considered “cis”. If they come off opposite sides it is “trans”
Examples:
1. 2 - butene (cis-2-butene)
H
H
C C H
H
H
C
H H
C
H
2. 3-ethyl-4,5-dimethylhexa-1,2-diene
(Note the "di" for two double bonds and the numerical positioning of the two double bonds)
H
3
C C CH
2
H
3
C
H H
CH
2
CH
3
CH
3
3. Propyne (1-propyne)
H
3
C C CH
4. 3-butylpenta-1,4-diyne
(Remember that the parent chain must include all multiple bonds.
H C
C CH
2
CH
2
CH CH
2
CH
3
C
CH
Note: Alkenes and alkynes are known as unsaturated hydrocarbons.
Cycloalkanes
Sometimes carbon molecules form rings. Single bonded rings are known as cycloalkanes. In naming them:
1. If it is a simple ring without any substituents simply name the compound with the word cyclo in front of it.
Example: Cyclopentane
H H
H
C
H
C C
H H
C C
H H
This may also be written in shorthand as
Note that everyplace a corner exists denotes a carbon (you must mentally fill in the hydrogens based on the knowledge that carbon has four bonds total.)
Example 2: Cyclohexane
If substituents exist on the ring structure, number the ring carbons in such a way as to create the smallest number(s) for branch locations:
Example 1 : 1-ethyl-2-methylcyclobutane
NOT 1-ethyl-4-methylcyclobutane or any other numbering system.
H
2
C CH
2
CH CH
CH
3
H
3
C CH
2
Example 2 : 1,2-dimethylcyclopropane
CH
3
CH
3
Note: A "prop" is the minimum number of carbons capable of making a ring structure.
Functional Groups:
Sometimes other specific groups of atoms (often containing oxygen and nitrogen in addition to carbon) may be part of the molecule. These easily identifiable
"groupings" are called functional groups.
Some examples are:
Alcohol
R O H
-ol suffix
Carboxylic acid -oic acid suffix
R C O H
O
Ketone
O
-one suffix
R C
Amine
H
3
C N
R
2
-amine suffix
R
2
R
1
Ester
R
1
C
-oate suffix
O R
2
O
There are several others in addition to these. Note that the R
1
, R
2
, etc. are placeholders representing carbon groups. R
1
doesn't necessarily have to have the same number of carbons in the chain as R
2
and so on.
Examples:
1. Ethanol
CH
2
H
H
3
C O
2. Ethanoic acid (acetic acid)
H
3
C C O H
O
3. Ethyl amine
CH
2
H
3
C N
H
H
4. Methyl ethanoate
H
3
C C O CH
3
O
Chemistry
Aromatic Compounds
Aromatic compounds are so named because the first ones identified had very strong characteristic odors.
Although it is now known that not all of these types of compounds have odors, the name continues to be used.
All aromatic compounds contain a structure known as a benzene ring . The structure for benzene was first discovered in 1865 by August Kekulé. He is said to have had a dream of a serpent biting its own tail.
From that he derived the ring structure of benzene.
Benzene is a six-carbon ring with alternating single and double bonds. More specifically, it is a resonance structure in which the double bonds form a “doughnut ring” of delocalized electrons above and below the plane of the carbon ring. For this reason, benzene can be represented as:
Or
Benzene in its pure form is a known carcinogen, but chemically is fairly stable. Its structure is found in a great number of products and medications.
Alkyl-substituted benzenes are sometimes referred to as arenes . If the alkyl group has less than six carbons, it is named as a substituted benzene, otherwise the benzene ring is considered the substituent and is called a phenyl (fen’-nil) group.
Some benzene containing compounds have special trivial names. For instance:
Toluene Phenol Analine
CH
3
OH
NH
2
The point of attachment for these compounds is always considered carbon 1 when numbering the ring.
On a compound such as xylene (see below) the terms ortho, meta and para are used to indicate the relative positions of the methyl group substituents on the benzene ring.
CH
3
Ortho-Xylene
(o-Xylene)
H
3
C
(1,2-dimethylbenzene)
CH
3
(m-Xylene)
H
3
C
Meta-Xylene
(1,3-dimethylbenzene)
CH
3
Para-Xylene
(p-Xylene)
H
3
C
(1,4-dimethylbenzene)
Benzene with a methyl group between it and the parent chain (when acting as a branch) is known as a benzyl group (see figure at right).
CH
2
H
3
C
Examples of Benzene molecules:
CH
3
4-isopropyl-3-propyltoluene
CH
3
2,4,6-trinitrotoluene (TNT)
CH
3
Diazepam (Valium)




