Bio-Medical Waste Management Satish Sinha
advertisement

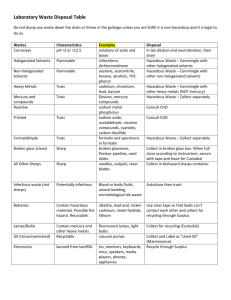
Bio-Medical Waste Management Satish Sinha History of medical waste • Medical Waste Tracking Act in US • I Draft Rules in India–1995 • Final Rules in 1998, 2 amendments and 5 guidelines • Evolution of Rules and Practices through National Experiences • National Guidelines on BMW, Guidelines on Incineration, CTFs, Immunization Waste and Mercury Various networks NGOs • Health Care Without Harm (HCWH) Injection safety: • SIGN (Safe Injection Global Network) Anti-incineration: • GAIA (Global Anti Incinerator Alliance) Mercury • Zero Mercury World Health Assembly • Patient safety Stockholm Convention on Persistent Organic Pollutants • an international environmental treaty • aims to eliminate or restrict the production and use of persistent organic pollutants (POPs). • entered into force on 17 May 2004 with ratification by 128 and 168 signatories. Basel Convention • Control of Tran boundary Movement of Hazardous Wastes and Their Disposal • Minimize hazardous waste generation and dispose it nearest to the point of generation Environmental Regulations • • • • • • • • Environment Protection Act, 1986 BMW Rules 1998 Municipal Waste (Management and Handling) Rules, 2000 Atomic Energy Act Hazardous Wastes (Management & Handling) Rules, 1989 E-Waste Rules Batteries (M&H) Rules 2001 Manufacture, Storage and Import of Hazardous Chemicals rules, 1989 Patient safety and Bio-medical waste management • In 2002 World Health assembly, passed a resolution calling member states to work for safety of Patients. • In Oct. 2004, World alliance for Patient safety was formed, who have identified certain challenges in relation to safety of patients. First Challenge is “Clean care is Safer Care” (2005) • A formal pledge committing to address health care-associated infection in the country was signed by Government of India. Priority areas for Patient safety • Safe clinical practices and hand hygiene • Safe Surgical practices • Blood Safety • Safe Injections Practices • Health Care Waste Management Rules and guidelines are available but implementation is very poor. Lack of training or poor training is also a factor. It has not been given the due priority by most of the states and dedicated budget is required. All states should focus on this. Health care associated infections • Complicate between 5-10% of admissions in acute care hospitals in industrialized countries • It is estimated that this risk is up to 20 times higher in developing world • At any given time, 1.4 million people worldwide suffer from HAI, and at least 50% of HCAI are preventable. Unsafe injections • India contributes to 25%-30% of the global injections (WHO, 1999) • Annual injection usage ~ 3 – 6 billion, of this nearly two-thirds (62.9%injections) unsafe India CLEN Study 2002-04 Why Follow Universal Precautions • The prevalence rate of blood born diseaseHepatitis B 38/1000, HIV 7/1000 (NACO 1993) • Difficult to test each patient • NSI and other sharp injuries are the key Canadian health issue, affecting 70000 people per year and costing around dollar 140 million. • A safety programme at Toronto Hospital achieved 80% reduction in injuries within an year. What is this concern for? • • • • • • • Infectious waste (solid and liquid) Sharps waste Cytotoxic waste Pharmaceutical waste Radioactive waste Chemicals and disinfectants Pressurised containers BMW Rules and Key Actors • • • • • • Notified in 1998 Concept of PPP model Identified technologies and standards CPCB SPCB Department of Health Headline of presentation to come here (on slide master) Know your waste 80 70 60 50 40 30 20 10 0 Infectios Hazardous General Waste Treatment & Disposal System Category Waste category Treatment Category 1 Human anatomical waste Inc/burial Category 2 Animal waste Inc/burial Category 3 Microbiology & biotechnology Inc/alternate waste Category 4 Waste sharps Category 5 Discarded medicines, cytotoxic drugs Category 6 & 7 Solid waste Category 8 Liquid waste Autoclaving, microwaving & mutilation for category 7 Disinfection Category 9 Incineration ash Landfill Category 10 Chemical waste Drain/secured landfill after treatment Disinfection & autoclaving/microwaving/shre dding & mutilation Inc/landfill Schedule II Colour coding Type of Container I Waste Category Human, animal, microbiology, soiled waste Treatment options as per Schedule I Yellow Plastic bag Incineration/deep burial Red Disinfected Microbiology, solid & Autoclaving/Microwa container/ plastic bag soiled waste ving/Chemical Treatment Blue/White translucent Plastic bag/puncture proof container/Sharps Blaster Waste sharps & solid waste Autoclaving/Microwa ving/Chemical Treatment & destruction/shreddin g Black Plastic bag Discarded medicine, cytotoxic drugs, incineration ash & Chemical waste Disposal in secured landfill Bio-medical waste and technology • Technology is only a fraction of the solution. • Major components of waste management are: o Segregation of waste o Waste minimisation o Reducing use of hazardous substances or processes o Waste Audit Approved treatment methods • Autoclave • Chemical disinfection • Hydroclave • Microwave • Incineration • Any other technology after CPCB approval In house management of waste • 1.Survey 2.Meeting with the heads of all the departments 3.Forming a waste management committee 4.Rounds of wards to see the functioning 5.Creating a model ward 6.Suggest equipment procurement 7.Formal training for all the nursing staff 8.Implementing the system throughout the hospital Right Technology Medical waste management is 80% segregation and 20% technology • Incineration: Pathological Waste and Body Parts , no chlorinated plastics • Autoclaving: All except body parts and pathological waste • Microwaving: All except pathological waste and metals • Chemical: Mainly plastics Of site management of wasteCentralized Facilities Draft Guidelines on Common facilities•Treatment facilities- 90% non-burn, 10% waste- burn •Limits incineration to Categories 1&2 •Atleast 1 Km from residential areas. Acceptable in industrial area •One operator allowed to cater upto 10,000 beds, situated within 150 km radius •Segregation is the role of generator; operator can report mixing of waste to the prescribed authority Medical waste in India: 2006-2009 2006 HCF Waste Incinerator 2008 2009 Total Number of Healthcare facilities 73975 129511 Number of HCFs linked to CTFs / own facility 34001 116080 Number of facilities where waste is not being treated 39974 13431 Percentage of total facilities with no type of treatment mechanism 54% 10% Bio-medical waste generated /day 319453 kgs 413500# 414956# Bio-medical waste treated /day 143952 kgs 295270 291983 Bio-medical waste not treated /day- 175501 kgs 113719 Percentage of Bio-medical waste untreated /day 55% 28% Total incinerators in the country 436 547 Incinerators with APCDs 207 250 Incinerators without APCDs 229 297 Total Number of Violations 24,412 13037 HCF issued Show cause notices 14898 Hurdles in Implementation Issues of Capacity Low priority Resource Allocation Fixed Mindset Injection safety, chemical safety and waste management issues yet to find space in development planning At the SPCB level Capacity and resource Monitoring and control Transparency of processes Hierarchy of control Independent audits Awareness of community Increasing outreach of centralized facility to rural areas At the Hospital level Mindset issues Involvement of senior management Resource availability and prioritising Government Hospitals biggest defaulters Capacity Building Implementation bottlenecks Responsibility fixing Monitoring and Accreditation Periodic Waste audits wrt economics At the CTF level Untrained Staff Poor maintenance of equipment Effluent Treatment Plants Maintenance of records No power back ups Closed door, non transparent Differential charges Flawed systems Profit driver Need for accreditation Way Forward Resource allocation for waste management Maintaining a pool of trainers at block/ district levels Stakeholders involvement Incorporation into curricula of medical, nursing and paramedical colleges Up gradation to latest developments in BMW management Waste minimizations policy Appropriate technology selection Pro-environment procurement policy Emerging Issues Mercury First mercury documentation in healthcare in 2004: 3 kg/ hospital/year Public notices by DPCC Mercury phase-out committee formed by DHS Delhi hospitals to phase out mercury No new mercury equipment procurement in Delhi government hospitals HCEs aiming for ISO/ NABH to phase out mercury Emerging Issues Injection Safety Increased attention by hospitals Fines on unattended needles No to recapping Reporting of needle stick injury and follow up Chemical Safety Monitored use of Glutaraldehyde, formaldehyde, benzene, cytotoxic drugs etc. Thank You Toxics Link H-2, Jungpura Ext. New Delhi 110014 011-24328006, 24320711 info@toxicslink.org www.toxicslink.org