Methyl Orange Synthesis: Diazotization-Coupling Reaction
advertisement

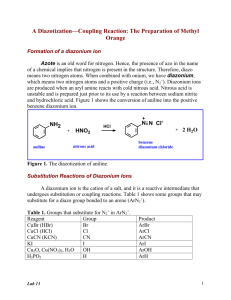
A Diazotization—Coupling Reaction: The Preparation of Methyl Orange Formation of a diazonium ion Azote is an old word for nitrogen. Hence, the presence of azo in the name of a chemical implies that nitrogen is present in the structure. Therefore, diazo means two nitrogen atoms. When combined with onium, we have diazonium, which means two nitrogen atoms and a positive charge (i.e., N2+). Diazonium ions are produced when an aryl amine reacts with cold nitrous acid. Nitrous acid is unstable and is prepared just prior to its use by a reaction between sodium nitrite and hydrochloric acid. Figure 1 shows the conversion of aniline into the positive benzene diazonium ion. NH2 + aniline HNO2 HCl nitrous acid N N Cl+ 2 H2O benzene diazonium chloride Figure 1. The diazotization of aniline. Substitution Reactions of Diazonium Ions A diazonium ion is the cation of a salt, and it is a reactive intermediate that undergoes substitution or coupling reactions. Table 1 shows some groups that may substitute for a diazo group bonded to an arene (ArN2+). Table 1. Groups that substitute for N2+ in ArN2+. Reagent Group CuBr (HBr) Br CuCl (HCl) Cl CuCN (KCN) CN KI I Cu2O, Cu(NO3)2, H2O OH H3PO2 H Lab 13 Product ArBr ArCl ArCN ArI ArOH ArH 1 A generalized equation for a substitution reaction is shown below in which the reagent can be any reagent in Table 1 and X any group. N2+ X reagent Coupling Reactions of Diazonium Ions Compounds such as aniline and phenol, which contain strong electron donating groups (e.g., -OH and –NH2) that activate the ortho and para positions on a benzene ring, can undergo coupling reactions with a diazonium ion. A coupling reaction is one in which two aryl rings are joined by an azo group. These coupling reactions usually occur at the para position of the o,p director. Figure 2 shows the coupling of benzene diazonium ion with phenol at the para position of phenol. N N Cl- + H OH phenol benzene diazonium chloride N N OH + HCl diazo product Figure 2. Coupling reaction with phenol. Aniline may serve as the substrate for the formation of a diazonium ion as shown in Figure 1, and it may serve as the substrate for a coupling reaction with the diazonium ion as shown in Figure 3. N N Clbenzene diazonium chloride + H NH2 aniline N N NH2 + HCl diazo product Figure 3. Coupling reaction with aniline. The mechanism for a coupling reaction is shown in Figure 4. The reaction is an electrophilic aromatic substitution reaction; therefore, the mechanism is similar Lab 13 2 to that for the nitration of benzene (i.e., it is a two-step reaction in which the first step is rate determining). N N+ H benzene diazonium ion OH H N N slow OH phenol fast N N OH diazo product Figure 4. Mechanism of coupling. The Experimental Reaction In this experiment, sulfanilic acid is the primary aryl amine that will be diazotized, and N,N-dimethylaniline is the ring-activated benzene derivative to which the diazonium ion will couple in the para position. Figure 5 shows the structures of aniline, N,N-dimethylaniline and sulfanilic acid. Both reactants are derivatives of aniline. NH2 H3C N CH3 NH2 SO3H aniline N,N-dimethylaniline sulfanilic acid Figure 5. Aniline derivatives. Lab 13 3 Sulfanilic acid contains a sulfonic acid group (SO3H) and an amino group (NH2); therefore, it undergoes an internal acid-base reaction to form a di-ionic species. Sodium carbonate acts as a base and deprotonates the quaternary ammonium ion. The ensuing sulfonate salt is diazotized with cold nitrous acid, as shown in Figure 6. NH2 NH3 H+ transfer N2+ NH2 Na2CO3 HNO2 cold SO3- SO3H SO3- SO3- diazotized sulfanilic acid sulfanilic acid Figur e 6. Diazotization of sulfanilic acid. The diazotized sulfanilic acid couples with N,N-dimethylamine, as shown in Figure 7. Adjustment of the pH to about 4 by the addition of NaOH yields an orange product known as methyl orange. Methyl orange is the well-known acid— base indicator and is an example of an anil, an aniline-based dye. If the pH is not properly adjusted a dark red compound helianthin might be isolated instead of methyl orange. -O S 3 N N NaO3S N(CH3)2 + diazotized sulfanilic acid N N N(CH3)2 NaOH N N H N(CH3)2 H+ transfer N,N-dimethylaniline methyl orange Lab 13 -O S 3 -O S 3 H N N N(CH3)2 helianthin (red) 4 Procedure Preparation of the Diazonium Ion of Sulfanilic Acid 1. Weigh 0.6-g anhydrous sodium carbonate and transfer it to a 25-mL Erlenmeyer flask. 2. Add 5-mL water to the Erlenmeyer and dissolve the solid completely. 3. Weigh 0.2-g sulfanilic acid and transfer it to the Erlenmeyer. 4. Fill a 150-mL beaker half full of water and heat to boiling on a hot plate. 5. Immerse the Erlenmeyer flask in the hot-water bath, using a pair of tongs or test tube holder. 6. After all of the sulfanilic acid dissolves completely, remove the Erlenmeyer flask and allow it to cool to room temperature on the bench top. 7. Weigh 0.08-g sodium nitrite, NaNO2, and transfer it to the cooled Erlenmeyer flask; stir the solution until the solid dissolves. 8. Cool the 25-mL Erlenmeyer flask in an ice-water bath for 10 min. 9. Add five drops of concentrated hydrochloric acid to the Erlenmeyer while it remains in the ice bath. The diazonium salt of sulfanilic acid precipitates as a finely divided, white solid. Keep the suspension in the ice bath until needed in Step 2 below. Preparation of the Diazo Dye Methyl Orange 1. Add four drops of N,N-dimethylaniline and two drops of glacial acetic acid to a small test tube. 2. Transfer the solution of N,N-dimethylaniline from Step 1 to the 25-mL Erlenmeyer flask in the ice bath. Keep the Erlenmeyer in the ice bath. 3. Stir the mixture vigorously with a stirring rod. A red precipitate of helianthin forms. 4. Keep the Erlenmeyer flask in the ice bath for 10 min. Lab 13 5 5. Slowly add 1.5-mL 10% sodium hydroxide to the Erlenmeyer while it remains in the ice bath. 6. Remove the Erlenmeyer from the ice bath, dry it with a paper towel, place it on the hot plate and heat the mixture to boiling. 7. Remove the Erlenmeyer from the hot plate and add 0.5-g sodium chloride to the hot solution. 8. Allow the Erlenmeyer to cool on your bench for two min., and then place the flask in an ice-water bath. After crystallization is complete, collect the dye on a pre-weighed filter paper in a Büchner funnel. 9. Rinse the Erlenmeyer flask with 3 mL of an ice-cold, saturated solution of sodium chloride; swirl the Erlenmeyer and pour this solution into the Büchner funnel. 10. Allow the dye to dry on its filter paper, and then re-weigh the filter paper to determine the yield of methyl orange. Calculate the percent yield. If your next experiment is an unknown sugar, continue with the following procedure, otherwise skip to Step 7 below. Preparation of Solution for Specific Rotation (To be conducted one week before observing the rotation) 1. Obtain an unknown sugar, show the instructor the number of the unknown and record it in your lab notebook. 2. Keep about 0.3 g of the unknown for next week’s experiments. 3. Tare a 25-mL volumetric flask on an analytical balance that measures to four decimal places (instrument room). 4. Transfer the remainder of the unknown sugar (about 3 g) to the volumetric flask and record the mass of the unknown. 5. Add distilled water to the volumetric flask until the water level reaches about 2 cm below the etched mark on the flask. Lab 13 6 Water will be added to the mark at the next lab meeting. The sugar will undergo mutarotation during this time. 6. Stopper the volumetric flask, mark it with your initials, and save it in the designated location until next week. 7. Clean the glassware, ensure the balance area is clean and the balances are turned off. Ensure the sinks are clean with no solid materials left in them. Lab 13 7 Diazotization Questions Stu No___ Sec___ Last name___________________________, First______________________ 1. Draw the structure of m-bromo-N-ethylaniline. 2. Which of the following rankings correctly shows the order of increasing acidity for benzoic acid, benzensulfonic acid, ethanol and phenol? __A. phenol < ethanol < benzoic acid < benzenesulfonic acid __B. ethanol < phenol < benzenesulfonic acid < benzoic acid __C. ethanol < phenol < benzoic acid < benzenesulfonic acid __D. ethanol < benzoic acid < benzenesulfonic acid < phenol 3. Which of the following answers applies to the electron-withdrawing or electrondonating power of an amino group and a hydroxyl group? __A. both are electron-donating groups. __B. both are electron-withdrawing groups. __C. the amino group is electron withdrawing and the hydroxyl group donating. __D. the amino group is electron donating and the hydroxyl group withdrawing. 4. Draw resonance structures that support your answer to problem 3. NH2 OH 5. Write an equation for the diazotization of aniline. Lab 13 8 Complete the following equations by inserting the reagent or product. 6. N N + 7. H2O and copper salts N N + 8. N N + KI OH 9. N N + NH2 N N 10. Lab 13 + 9