CHM 112 NAME ____________________________________ GAGE
advertisement

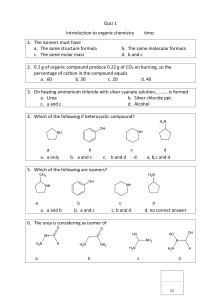
CHM 112 GAGE SPRING 2002 NAME ____________________________________ DATE_____________________________________ EXAM 5 1. a. Join the two amino acids below to form one of two possible dipepetides. O O H2N CH C (5) OH H2N CH C OH CH 2 CH 2 CH 2 C O OH b. Show by means of equations how glycine can act as a buffer. (5) O H2N CH C OH H c. What is the tertiary structure of a protein and how is it maintained? (5) 2. At the end of this exam you will find a copy of the Krebs Cycle or the Tri-Acid Cycle. Steps and compounds in this process have been numbered. Use these numbers to interpret and respond to questions below where appropriate. When answering about a reaction, select the clockwise direction unless otherwise indicated. (16) a. What is the source of the pyruvate (1)? ________________________ b. List the functional groups on molecule 4. ________________________ c. What type of reaction is happening at 5? ________________________ d. List any steps where the electron transport chain will be engaged. ________________________ e. After which step is the pyruvate totally oxidized? ________________________ f. What type of reaction is occurring at 12? ________________________ g. Which step is exothermic? ________________________ h. What type of reaction is occurring at step 16? ________________________ i. Explain why this metabolic pathway is shown with multidirectional arrows. (4) 3. a. Explain how an enzyme is able to increase the rate of a biochemical reaction. (5) b. What does lactase dehydrogenase do? (2) c. Sketch a graph of reaction rate as a function of substrate concentration and explain why graph has the shape it does. (4) d. Explain from a structural perspective why enzymes have a optimal temperature of operation. (4)