Molecular Geometry and Valence Bond / Molecular Orbital Theory Chapter 10
advertisement

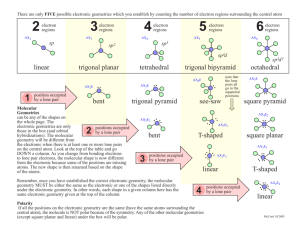
Molecular Geometry and Valence Bond / Molecular Orbital Theory Chapter 10 Molecular Shapes • Sugars and sweeteners taste sweet because they fit into spots on the tongue that trigger that response • Artificial or ‘no calorie’ sweeteners fit in the same place but do not get metabolized as quickly or at all… meaning they pass through fast and do not absorb VSEPR Theory • The Valence Shell Electron Pair Repulsion (VSEPR) Theory – States that electron pairs (defined as lone pairs or single, double, triple bonds, and even single e-’s) repel one another and want to push as far away as possible – Affects the shape (molecular geometry) Shapes • The preferred shape of a molecule is the one that allows maximum distance between electrons (bonding and lone pairs) • Depends on 1. How many come off the central atom(s) 2. How many lone pairs / bonding pairs TWO electron Groups • LINEAR: Maximizes separations by 180⁰ • Draw out BeCl2 • CO2 THREE electron Groups • Trigonal planar geometry: maximum separation is ~120⁰ • Draw BF3 • Draw CH2O – Notice the bond angles (ok to round to 120⁰ FOUR electron Groups • Tetrahedron geometry – maximum bond angle is 109.5⁰ • Draw CH4 FIVE electron Groups • Trigonal Bipyramidal Geometry – 2 bond angles for maximum separation: 90 ⁰ & 120⁰ • Draw out PCl5 • “Axial chlorines” (top and bottom) vs the three “equatorial Chlorines” SIX electron Groups • Six electron groups around a central atom assume an octahedral geometry • Draw (think about) SF6 • Maximum bond angle is ALL 90⁰ Practice • Determine the molecular geometry of NO3– Draw it out first – Resonance ? – How many bonding sites?? • Three… what does this tell about its shape? Bonded vs Un-bonded electrons • The previous shapes were all “bonded” electron groups • Think about what happens with un-bonded electron pairs (lone electron pairs) • VSEPR theory says these are also greatly (-) and will push away and repel from one another in a similar fashion – Only they create different shapes because there is not an atom to “see” on the lone pairs • Nonbonding electrons still push (very, very negative) • They still have repulsions and according to the VSEPR theory they will migrate as far away as possible to alleviate this stress REPULSIONS Lone pair-lone pair > lone pair-bonding pair > bonding pair-bonding pair Most Repulsive Least Repulsive • Think about how this can affect bond angles • The more repulsive (the greater they push away) the greater the bond angle FOUR electron groups with ONE LONE PAIR • Think about ammonia – There are 4 electron groups (3 bonded and 1 lone) • So, push them apart just like a tetrahedron… but there is nobody bonded on one side • This ‘push’ creates a pyramidal shape (trigonal pyramidal) Trigonal Pyramidal FOUR electron groups with TWO LONE PAIRS • Think about Water – There are 4 electron groups (2 bonded and 2 lone) • So, push them apart just like a tetrahedron… but there is nobody bonded on two sides • This ‘push’ creates a BENT shape Bent Molecular Geometry FIVE electron groups with ONE LONE PAIR • Draw out / Consider SF4 • In order to get these as far away as possible: SEESAW Geometry FIVE electron groups with TWO LONE PAIRS • Draw out / Consider BrF3 • In order to get these as far away as possible: T-SHAPED Geometry FIVE electron groups with THREE LONE PAIRS • Draw out / Consider XeF2 • In order to get these as far away as possible: LINEAR Geometry SIX electron groups with ONE LONE PAIR • Think about BrF5 – There are 6 electron groups (5 bonded and 1 lone) • So, push them apart just like a octahedron… but there is nobody bonded to one of the sides • This ‘push’ creates a SQUARE PYRAMIDAL MOLECULAR GEOMETRY SIX electron groups with TWO LONE PAIRS • Think about XeF4 – There are 6 electron groups (4 bonded and 2 lone) • So, push them apart just like a octahedron (AGAIN)… but there is nobody bonded to TWO of the sides • This ‘push’ creates a SQUARE PLANAR MOLECULAR GEOMETRY Summary of VSEPR Theory • The geometry of a molecule is determined by the number of electron groups on the central atom(s) • The number of e- groups can be determined from the Lewis structure of the molecule. If the molecule has resonance structures, use one of them – doesn’t matter which one • Each of the following counts: lone pair, single bond, double bond, triple bond, or a single electron Summary (cont’d) • The geometry of the e- groups is determined by minimizing their repulsions: Lone pair-lone pair > lone pair-bonding pair > bonding pair-bonding pair • Bond angles will vary, the presence of lone pairs will usually make bond angles smaller than the ideal angle of the particular geometry Page 414-415 Copy this table On a piece of Computer paper (provided) Review sheet / Study guide / Summary for Molecular geometry Quick Practice • Which of the following statements is always true according to VSEPR theory? a) The shape of a molecule is determined by repulsions among bonding electron groups. b) The shape of a molecule is determined by repulsions among nonbonding electron groups c) The shape of a molecule is determined by the polarity of its bonds d) The shape of a molecule is determined by repulsions among all electron groups on the central atom VSEPR Theory: Predicting Shapes 1. Draw the Lewis structure 2. Determine the total number of electron groups around the central atom 3. Determine the number of bonding groups and the number of lone pairs around the central atom 4. Use Table 10.1 to determine the electron geometry and molecular geometry Polarity • As we discussed in chapter 9, bonds can be polar (unequal sharing of electrons, EN) • Entire molecules can be POLAR!! Polarity • Bonds are easy… just look at the differences in electronegativity • For molecules, there must be a ‘pull’ in different directions, but not equal and opposite WE STILL NEED TO USE OUT TABLE FROM CHAPTER 9! Nonpolar Molecules • Think about two kids playing tug-o-war… – If they are equal in strength, there will be no net dipole moment – They cancel out – Go nowhere Polar Molecules • Consider the same two kids pulling… if now instead, one kid pulls up and one kid pulls to the left… there WILL be a net movement (they will not cancel out In summary, to determine whether a molecule is polar: • Draw a Lewis structure, determine the molecular geometry • Determine whether the molecule contains polar bonds • Determine whether the polar bonds add together to form a net dipole moment (add up vectors) – what is a vector??? Vector Addition • Refer to page 420 for VECTOR ADDITION • One dimension = two kids pulling left and right (left / left OR right/ right) • Two dimensions = pulling up and to the right (in different directions, off of the same plane) Page 421: Practice • Determine whether NH3 is polar 1. Draw the Lewis Structure 2. Determine where the molecule has polar bonds 3. Determine whether the bonds add together to form a net dipole moment • The three dipole moments sum to a net dipole moment. The molecule is POLAR! PRACTICE #2 • Determine whether CF4 is polar. Interactions • Nonpolar and Polar molecules DO NOT MIX – Think about water (polar) and oil (nonpolar) – They will separate and keep their distance • Polar molecules interact STRONGLY with other polar molecules – So when you mix P and NP together, the P will group and bunch up while the NP stay away and will not penetrate the group Page 422 • Chemistry in your day – How Soap Works • Read through this excerpt and answer the question in your notes – Consider the detergent molecule below (in your book). Which end do you think is polar? Which end is nonpolar? Hybridization • Hybridization is a mathematical preocedure in which the standard atomic orbitals are combined to form new atomic orbitals called Hybrid Orbitals • It is essentially adding two orbitals together SP3 hybridization ___ ___ ___ ___ Pi and Sigma bonds • A pi (π) bond is the overlap between the halffilled p orbitals overlap side-by-side • A sigma (σ) bond is the overlap end-to-end • A pi (π) bond is formed after the sigma bond • A sigma (σ) bond is always the FIRST BOND formed https://www.youtube.com/watch?v=Y cSPPKESpwc • Short unit… – Molecular shapes through the pi / sigma bonds