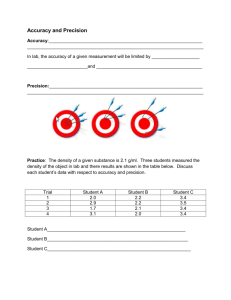
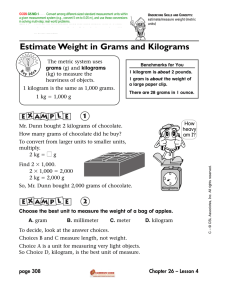
Name: ___________________________________ Date: _________________ Period: ________
advertisement

Name: ___________________________________ Date: _________________ Period: ________ Define the following terms: 1. Absolute Zero – 2. Accepted Value 3. Accuracy – 4. Celcius Scale – 5. Density – 6. Error – 7. Experimental Value – 8. Gram – 9. International System of Units (SI) – 10. Kelvin Scale – 11. Kilogram – 12. Liter (L) – 13. Meter (m) – 14. Percent Error – 15. Precision – 16. Qualitative Measurement – 17. Quantitative Measurement – 18. Significant Figure – 19. Scientific Notation – 20. Factor-Label Method – Bannister Name: ___________________________________ Date: _________________ Period: ________ 21. Conversion Factor – 22. Temperature – 23. Heat – 24. Volume – 25. Weight – 26. Mass – 27. Atomic Number – 28. Mass Number – 29. Mole – 30. What is the difference between weight and mass? 31. What is the difference between accuracy and precision? Can you be precise without being accurate? 32. Why are units so important in chemistry? How do they “walk you through” a conversion problem? 33. Name three possible sources of Error in the Laboratory. 34. When are zeros significant in measurements?? Give ONE example to illustrate EACH of our rules. Bannister