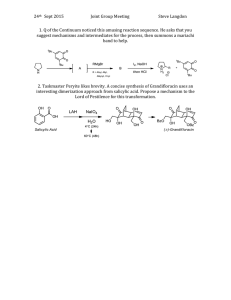
AB INITIO AND DFT STUDIES ON THE V.B. Singh
advertisement

Jai Bajrang Bali Ki JaiHo AB INITIO AND DFT STUDIES ON THE SPECTROSCOPY AND PHOTOPHYSICS OF ANTHRANILIC AND SALICYLIC ACID V.B. Singh Department of Physics Udai Pratap Autonomous College Varanasi-221002 , India 1. INTRODUCTION • • • • The PHOTOINDUCED Excited-State Intramolecular Proton/Hydrogen atom Transfer [ESIPT/ESIHT] process is one of the most elementary photoreactions observed in nature. To gain experimental and theoretical understanding of the mechanism of ESIPT/ESIHT in complex biological systems and practical applications , it is desirable to study simple systems , such as salicylic and anthranilic acids. It is nowdays well established for many ESIPT systems that the proton (or hydrogen) transfer is an extremely fast process . The femto second rise time of the red-shifted fluorescence provides a strong argument for an essentially barrierless process in the lowest excited singlet state. The photophysics of the ESIPT reaction has most frequently been discussed in terms of a tautomeric form in the excited state potential energy (PE) surface due to transfer of a H-atom along a preexciting intramolecular H-bond. Salicylic acid (SA) has served as a model compound for the experimental and theoretical investigation of the excited state intramolecular hydrogen - transfer . The conclusion that hydrogen-atom transfer occurs in salicylic acid is based on the early work of Albert Weller[1]. Weller postulated that proton transfer occurs in methyl salicylate to explain the dual fluorescence observed in the the emission spectrum of this molecule. Weller believed that the excited -state product was a zwitter-ion, leading to his description of this process as a proton transfer . Although calculations indicate that the excited-state dynamics of salicylic acid involve the motion of a neutral hydrogen atom rather than a proton. Anthranilic acid (AA) has a similar structure to that of Salicylic acid and both are known to form an intramolecular hydrogen bond . Anthranilic acid has this hydrogen bond between its amino and carbonyl groups, whereas salicylic acid form intramolecular hydrogen bond between hydroxyl and carboxylic groups. With respect to the internal rotation of the carboxyl group two conformational isomers [ rotamers ] are expected for each of the two molecules . 1. A.Weller Z.Electrochem. 60, 1144,(1956) SALICYLIC ACID ANTHRANILIC ACID SALICYLIC ACID ROTAMER ANTHRANILIC ACID ROTAMER SALICYLIC ACID DIMER-I SALICYLIC ACID DIMER-II SALICYLIC ACID DIMER-III ANTHRANILIC ACID DIMER-I ANTHRANILIC ACID DIMER-II ANTHRANILIC ACID DIMER-III Theoretical and experimental investigations on Anthranilic acid and Salicylic acid predicted that monomer of each molecule exists under an equilibrium condition with their corresponding cyclic dimer.It could be dimerise in different ways. AA and SA dimmers are perhaps the smallest aromatic systems in which both intra- and intermolecular hydrogen bonding exist and thus constitute an ideal model to study both modes of hydrogen transfer in a single system. Experimental information about structures of AA and SA and their Dimers have been reported so far by electronic spectroscopy and recently by IR spectroscopy by number of groups , however theoretical calculations are rather limited. The key to the structure determination is clearly to observe the intramolecular and intermolecular hydrogen bonded OH and C=O groups and IR spectroscopy should be the most suitable technique. In the present work (i) The ground state IR spectra of monomers and dimmers of Anthranilic and Salicylic acids have been studied by Ab initio and DFT calculations to explore the both intra- and intermolecular hydrogen bonding properties in the different conformers of the two molecules. Hydrgen bonding properties of Salicylic Acid and Anthranilic Acid are compared. (ii) The ab initio potential energy profile for the ground state and ground state rotamarisation in the each molecules were derived. (iii)The excited state energies and potential energy profiles were determined to explore photophysical properties of these systems ,using Time Dependent DFT and CIS calculations. (iv) Recent Studies on the Spectroscopy and photophysics of AA and SA are summarized and Excited-State Intramolecular Hydrogen atom 2.METHODOLOGY • The Ground state properties of each conformer of the Anthranilic Acid and Salicylic Acid have been investigated using Hartee-Fock (HF) and Density Functional Theory (DFT) methods using different basis sets upto 6-311g(d,p) level. The excited states have been studied using time dependent density functional theory With B3LYP funtional using different basis sets upto 631++g(d,p) level and configuration interaction including single excitation (CIS) method. All of the HF and DFT calculations reported here were carried out using the GAUSSIAN 03 . 3.Results and Discussion • • • • • A : Our DFT calculation predicts two IR bands at 3585* and 3251 cm-1 corresponding to OH stretching vibration in the ground state of the Salicylic Acid (SA). The IR band at 3585 cm-1 is clearly assigned to the free OH stretch of the carboxylic group and the band at 3251 cm-1 is attributed to the phenolic OH ( stretch ) , which is intramolecularly hydrogen bonded to the neighboring carbonyl group . Our results corroborates to the experimental values obtained from the high resolution fluorescence studies by Yahgi et al [1] and Bisht et al [2]. Thus the low frequency shift of the phenolic OH stretching due to the intramolecular hydrogen bond is roughly estimated to be 400 cm-1 , since the free OH stretching frequency of phenol is known to be 3657 cm-1 [ 3 ]. Our C=O stretching vibrational frequency of SA also shows a significant low frequency shift. On the otherhand , in the SA Rotamer , the phenolic OH stretch band was found at 3511 cm-1, while the free carboxylic OH stretch band was predicted again at 3585 cm-1 , as for the SA . These values also corroborates to the experimental values reported recently [1,2]. C=O stretching vibrational frequency of SA rotamer also shows very low shift in comparison to SA. Thse results clearly demonstrate the difference between the intramolecular hydrogen bonds in the SA and SA Rotamer. It is well expected that the intramolecular hydrogen bond in SA is stronger than that in SA Rotamer because the carbonyl O-atom is more basic than the carboxylic O . We have also performed HF and DFT calculations for the Harmonic vibrational frequncies of the isomer A of SA dimer.The IR band predicted in this conformer of SA at 3308 cm-1 is attributed to antisymmetric combination of the phenolic OH stretches and the band predicted at 2887 cm-1 is attributed to antisymmetric combination of the OH stretches in the (COOH)2 ring. These values also corroborates to the experimental values . In this respect, it is noticed that phenolic OH frequency shifted by 57 cm-1 , upon dimer formation. This result demonstrate that , When a strong intermolecular hydrogen bond is formed upon the dimerization , the nonbonding electrons of the carbonyl group are strongly attracted by the carboxylic OH group of the other unit , and it reduces the electron density between the phenolic OH and carbonyl O atom , resulting in the decrease of the intramolecular hydrogen bond length. Our DFT calculations for the ground state of Anthranilic Acid (AA) , predicted that NH2 Symmetric and AntiSymmetric Stretching at 3386* and 3556 cm-1. The corresponding values for the AA Rotamer was found to be at 3452 and 3580 cm-1 respectively . The OH stretch frequency [3592 cm-1] remains relatively unchaged after rotamerisation. These results , which corroborates to the experimental values , demonstrate that neither of NH2 stretch vibrational frequencies , of AA in its ground state , are strongly shifted due to intramolecular hydrogen bond , as found in the case of SA. So the intramolecular hydrogen bond in the AA is Weak , because the primary amines are known to be considerably weaker hydrogen-bond doners than the hydroxyl groups. DFT calculations for the ground state of AA Dimer-I predicts the NH2 symmetric and Antisymmetric stretch frequencies at 3400 and 3550 cm-1 which are very close to experimental values [4].These appear very close to the AA monomer ground state values[3386 and 3556 cm-1]. The AntiSymmetric [hydrogen-bonded ] OH stretch in the [COOH]2 ring was predicted at 2990 cm-1 , near to experimental value [5 ] . This extreme red shift is the characteristic of strong intermolecular hydrogen bonded dimers, as found in the case of SA. •The Calculated Results have been scaled by a factor of 0.9522. •References: 2. T. Yahgi, A. Fuzi, T. Ebata, and N. Mikami J. Phys.Chem. A 105,10673 (2001) 3. P.B. Bisht , H. Petek , K. Yoshihara and U. Nagashima J. Phys.Chem. A 103,5290 (1995) 4. T. Watanabe, T.Ebata, S.Tanabe and N.Mikami J. Phys.Chem. A 105,408 (1996) 5. C.A. Southern, D.H. Levy, G.M. Florio, A. Longarte and T.S. Zwier J. Phys.Chem. A 107,4032 (2003) 6. C.A.Southern, D.H. Levy, J.A.Stearns ,G.M.Florio, A.Longarte and T.S.Zwier J. Phys.Chem. A 108, 4599 (2004) Ground State (S0) IR of Salicylic acid at b3lyp/6-31g** Level 1756 3415 3765 IR of ground state Salicylic acid Rotamer , optimized at b3lyp/6-31g** 1826 3686 3765 Experimental Ground State IR SPECRA OF SA IN CONDENSED PHASE 1774 Ground state IR of Anthranilic acid at B3LYP/6-31G** level 3735/71 3556 Ground state IR of anthranilic acid Rotamer Optimized At b3lyp/6-31g** 1676.6 1806 3761 3626 Table.1 : Comparison of Calculated and Observed C=O and OH Stretch Frequencies of SA , SA Rotamer and SA Dimer A SA SA SA SA SA Rotamer Rotamer Dimer [ Obs.] [Calc] [Calc.] SA Dimer [Obs.] Vibrational Assignments [Calc.] [Obs.] 1668 - 1739 - - C=O stretching - - - - 2887 2900 OH stretching in (COOH) ring 3251 3248 3511 3530 3308 3295 Phenolic OH stretching 3585 3585 3585 3585 - - Carboxylic OH stretching Table.2 : Comparison of Calculated and Observed C=O and OH Stretch Frequencies of AA , AA Rotamer and AA Dimer A AA AA AA Dimer [Obs.] Vibrational Assignments [Obs.] AA AA AA Rotamer Rotamer Dimer [Calc] [ Obs.] [Calc.] [Calc.] 1684 - 1718 - - C=O stretching 3386 3394 3452 - 3400 3407 Symm NH2 stretching 3556 3542 3580 - 3550 3545 Asymm NH2 stretching 3591 3592 3585 - 2999 3000 Assymm.combinati on of Carboxylic OH stretch in (COOH) ring Rotational potential energy profile for Salicylic acid OH OH O O H H O 16 14 OH 12 H E (kcal/mol) 10 8 6 4 2 0 B3LYP/6-31G** -2 0 Relaxed scan 50 100 150 200 250 Dihedral angle (degree) 300 350 Rotational potential energy profile for Anthranylic acid OH 14 OH O O H 12 N H N H H 10 O E (kcal/mol) 8 OH H N 6 H 4 2 0 B3LYP/6-31G** -2 0 Relaxed scan 50 100 150 200 250 Dihedral angle (degree) 300 350 20 20 18 16 15 14 E (kcal/mol) 10 10 8 6 5 4 2 0 0 Salicylic acid Anthranilic acid 0 50 100 150 200 250 300 Salicylic acid Anthranilic acid -2 0 350 50 100 150 200 250 300 Dihedral angle (degree) Dihedral angle (degree) 20 Salicylic acid Anthranilic acid 18 16 14 E (kcal/mol) E (kcal/mol) 12 12 10 8 Dihedral scan keeping other parameters constatnt 6 4 2 0 0 50 100 150 200 Dihedral angle (degree) 250 300 350 350 Anthranilic acid ground state potential energy surface A.U N-H bond Distance Energy in kcal / mol E Potential energy surface of anthranilic acid CIS/-31G** Potential energy surface of anthranilic acid at CIS/6-31G** r [N-H] in angstrom S0 S1 S2 S3 Table: 3 Absorption maxima (in nm units) corresponding to vertical excitation energies of SA and SA rotamer and AA and AA rotamer Excited State SA TDDFT CIS CASSCF* MRCI* SA Rotamer Expt.a (B3LYP) Oscillator strengthb S1 297.0 218.57 203.4 216.9 335 0.0829 S2 242.4 179.53 170.2 189.6 0.0 S3 235.2 161.41 161.4 0.1328 S4 202.1 129.83 --0.2601 T1 381.8 346.66 262.9 266.3 0.0027 T2 347.6 327.38 253.8 260.1 0.0553 T3 389.9 283.10 217.7 228.9 0.00 T4 360.77 266.58 0.1686 a Obtained from the Ref. Yahagi et al. J.Phys.Chem.,105 ,10680 (2001) b Obtained from TDDFT method at B3LYP/6-311++g** level * Values taken from the Ref Maheshwary et al [ To be published] Excited State AA TDDFT (B3LYP) CIS Expt.a TDDFT (B3LYP) Expt.a Oscillator strengthb 290.2 241.8 227.10 202.23 371.2 347.8 288.7 -- 311 0.0830 0.0 0.1401 0.2600 0.0780 0.00 0.1456 -- AA Rotamer Oscillator strengthb TDDFT (B3LYP) Expt.a S1 323.34 235.43 349 0.1022 311.13 S2 254.47 186.75 0.0032 258.77 S3 245.77 164.09 0.00 244.85 S4 241.09 133.62 0.0332 237.75 a Obtained from the Ref. Southern et al J.Phys.Chem.,107 ,4032 (2003) b obtained from TDDFT method at B3LYP/6-311++g** level Oscillator strengthb 0.1070 0.0037 0.0002 Table: 3 Absorption maxima (in nm units) corresponding to vertical excitation energies of and SA dimer and AA dimer Excited SA Dimer I AA Dimer I State TDDFT Expt.a Oscillator TDDFT Expt.a Oscillator b (B3LYP) strength (B3LYP) strengthb S1 304.2 332 0.191 311.12 354 0.2484 S2 302.1 0.00 307.42 0.00 S3 287.9 0.00 296.75 0.00 S4 287.3 0.0123 296.37 0.0114 a The values obtained from the Ref. Southern et. al, J. Phys. Chem. A, 108, 4599 (2004) The above TDDFT values are obtained at B3LYP/6-311++g** level. Summary of Recent Experimental Theoretical studies : The conclusion that hydrogen-atom transfer occurs in salicylic acid is based on the early work of Albert Weller. Weller postulated that proton transfer occurs in methyl salicylate to explain the dual fluorescence observed in the the emission spectrum of this molecule. Weller believed that the excited -state product was a zwitter-ion, leading to his description of this process as a proton transfer . Although calculations indicate that the excited-state dynamics of salicylic acid involve the motion of a neutral hydrogen atom rather than a proton. Recent experimental and theoretical results have called into question whether H-atom transfer actually occurs in Salicylic Acid [ SA ]. Calculations by Sobolewski and Domcke [ 7,8 ] demonstrate that the excited-state potential energy surface of AA and SA have only a single minimum , which is closer to the enol form of AA(SA) than to Keto form.The geometry change that gives rise to the strongly stokes-shifted emission is the result of the rearrangement of atomes in the hydrogen bonded ring, which is termed a hydrogen-atom dislocation, rather than a full hydrogen-atom transfer. The IR spectrum of the S1 state of SA predicts a red shift of the phenolic OH stretching vibration by about 1400cm-1 whereas in AA a red shift of 500 cm-1 was predicted for the H-bonded NH vibration in the S1 state [ 8].The interpretation of Bisht et al[ ] regarding the supersonic jet spectroscopy of SA also suggests that only a partial transfer of the hydrogen atom occurs. Recently excited-state behaviour of the hydrogen –bonded dimer of anthranilic acid studied experimentally with combination of ultraviolet and infrared spectroscopy by Southern et al [ 6]. They concluded that the first excited singlet state of AA Dimer has a double minimum potential in which the electronic excitation is localised on one or other monomer in two wells. References: 7. A.L Sobolewski and W.Domcke Chem.Phys.232,257 ,(1998) 8. A.L Sobolewski and W.Domcke J.Phys. Chem. A 108, 10917 ,(2004) 4.CONCLUSION: 1.The Calculated IR Spectra of SA and AA in the OH/NH and C=O Stretching vibrational region clearly demonstrate the difference between the Rotamers in the each molecule and shows that the INTRAMOLECULAR HYDROGEN BOND in SA is considerably STRONGER than the INTRAMOLECULAR HYDROGEN BOND in AA. These results Corroborates to the recent high level experimental observations. 2.The HF and DFT Calculations predicted that SA and AA both are more stable than their corresponding Rotamers. In the each molecule SA and AA Dimer-I is more stable than Dimer II & Dimer-III ,Whereas Dimer –III is more stable than Dimer-II. 3.Our Results provides strong support for the Reliabilty of the TD-DFT/B3LYP Method for the calculation of Excitation energies of the excited singlet states of SA and AA , in comparison to the other Methods[ Like CIS,CASSCF,MRCI]. The trends of Excitation energies for the excited singlet states of the SA and AA Dimer-I are found to be same. 4.The recent experimental and theoretical investigations indicate that Although the Ground-state Hydrogen-Bonding properties of SA and AA [monomer] molecules differ , similar effect are observed in their lowest electronically excited singlet state S1. Both molecules exhibit a dramatic change in the strength of their intramolecular Hydrogen bonds upon electronic excitation. The experimental and theoretical results charaterised that the H-Atom is only dislocated in the S1 state of SA/AA monomers rather than transferred to form other tautomeric form. However this is an erea ripe for further investigation.