ATTACHMENT D. Informed Consent / Assent Authorization
advertisement
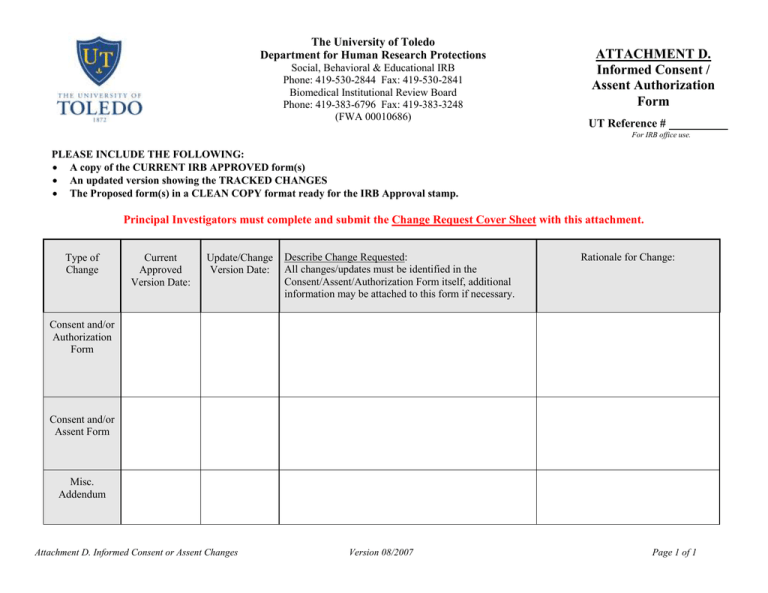
The University of Toledo Department for Human Research Protections Social, Behavioral & Educational IRB Phone: 419-530-2844 Fax: 419-530-2841 Biomedical Institutional Review Board Phone: 419-383-6796 Fax: 419-383-3248 (FWA 00010686) ATTACHMENT D. Informed Consent / Assent Authorization Form UT Reference # For IRB office use. PLEASE INCLUDE THE FOLLOWING: A copy of the CURRENT IRB APPROVED form(s) An updated version showing the TRACKED CHANGES The Proposed form(s) in a CLEAN COPY format ready for the IRB Approval stamp. Principal Investigators must complete and submit the Change Request Cover Sheet with this attachment. Type of Change Current Approved Version Date: Update/Change Version Date: Describe Change Requested: All changes/updates must be identified in the Consent/Assent/Authorization Form itself, additional information may be attached to this form if necessary. Rationale for Change: Consent and/or Authorization Form Consent and/or Assent Form Misc. Addendum Attachment D. Informed Consent or Assent Changes Version 08/2007 Page 1 of 1






