In-Class Group Activity #1 (Chem 101, Summer 2006) Name _________________________________
advertisement

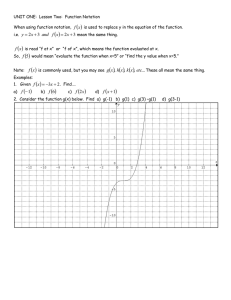
In-Class Group Activity #1 (Chem 101, Summer 2006) Name _________________________________ 1. Complete the following equalities: a) __________ mL = 1 L b) __________ km = 1 m c) __________ g = 1 g 2. a) Express 705.40 meters in scientific notation b) Round the same number to the nearest meter c) Express the rounded number in scientific notation 3. State whether each of the following is an exact or a measured number: a) A sample of Aluminum weighs 5.50 g b) A baby is 14 days old c) A grasshopper has 6 legs 4. Suppose that the mass of an unknown metal sample is measured by five groups of students (all on the same scale) with the following results: 5.203 g, 5.199 g, 5.208 g, 5.206 g and 5.205 g. It turns out that the true mass of the sample is 5.000 g. a) Are the students’ results accurate? Explain. b) Are the students’ results precise? Explain. c) Is the error in this set of experiments most likely due to student error or a problem with the scale? Explain. 5. How many miles is a 10 kilometer running race (1 km = 0.621 mi)? 6. Human body temperature is 98.6º Fahrenheit a) Express this temperature in degrees Celsius b) Express it in Kelvins 7. The mass of the sun is 1.989 x 1030 kg and the radius is 6.96 x 105 km a) Calculate the volume of the sun in km3 and express the answer with the correct number of significant figures (volume of a sphere = 4r3/3) b) Calculate the average density of the sun in kg/km3 (use correct sig. figs.)